The First Weight Loss Drugs
Long before Ozempic and Mounjaro, there were mitochondrial uncouplers. While deadly if not used with care, it might be time for them to make a comeback.
Poudrerie Nationale de Bassens, Bordeaux, France, during World War I.
Georges wasn’t feeling his usual self. Perhaps it was the late-summer heat that made shifts in the drying room at the Poudrerie near-intolerable,1 or the extra swig of brandy he had snuck on break while the overseer’s gaze was averted. While Georges normally held off the worst of his drinking until after work, he’d felt unusually thirsty today.
The malaise crept in after lunch. George’s arms hung heavily; he could hardly lift the cast steel shell casings to the filling stations. His heart beat faster than normal, and his breath grew short and labored.
Georges had been losing weight recently. His wife suspected overexertion — Georges had taken on extra shifts after some of his colleagues at the plant had fallen ill. The guns of the Western Front were ravenous, and the plant in Bassens supplied the explosive Mélinite for the artillery shells that held the Germans at bay.
Mélinite, also known as picric acid or trinitrophenol (TNP), was adopted by the French in 1887 and quickly became a national favorite. It was not hard to see why, as TNP was the first powerful explosive that didn’t regularly detonate in the barrels of artillery from the shock of firing. And yet, TNP alone is still dangerously volatile.2
To tame TNP, the French had learned to mix it with dinitrophenol (DNP),3 a yellow, musty-smelling explosive less sensitive to shockwaves. Before casting and filling artillery shells, French munitions workers would mix TNP and DNP, to be dried and ground into powder using mills like the one in Bassens in which Georges worked. This process released a fine dust that hung in the air and infiltrated workers’ lungs.4
After George’s ten-hour shift ended, he staggered out of the building. He was found only minutes later on a little river path by a sentry. Dripping with yellow-tinged sweat, Georges was hot to the touch, his eyes fixed on distant space. The sentry rushed him to the infirmary, where his temperature read 43 Celsius — 6 degrees above normal. The doctor had no time to prepare an ice bath: Georges’ heart stopped beating on arrival, his muscles as rigid as blocks of clay, fired from within.
Georges was one of several dozen workers who died like this between 1916 and 1918 in the French munitions factories. These deaths spurred a series of investigations into munitions intoxication. The French investigation was led by Roger Perkins, an American medic linked to the scientific attaché in Paris. Perkins visited sites in Bassens, Neuville, Saint-Fons, and Sorgues and recorded statistics on the chemical poisonings. He took a special interest in DNP, which was, in his words, “particularly a French explosive.” Buried in his report was an offhand comment about weight loss experienced by workers (like Georges) exposed to the chemical:
Workers claim that they have grown thin to a notable extent after several months' work in DNP. Many complain of general weakness with headache and dizziness, with moderate sweats, especially at night. A few days' rest are usually sufficient for a complete cure.
This observation, borne of accidental exposure, would eventually spur one of the first weight loss drug crazes — long before Ozempic and Mounjaro.
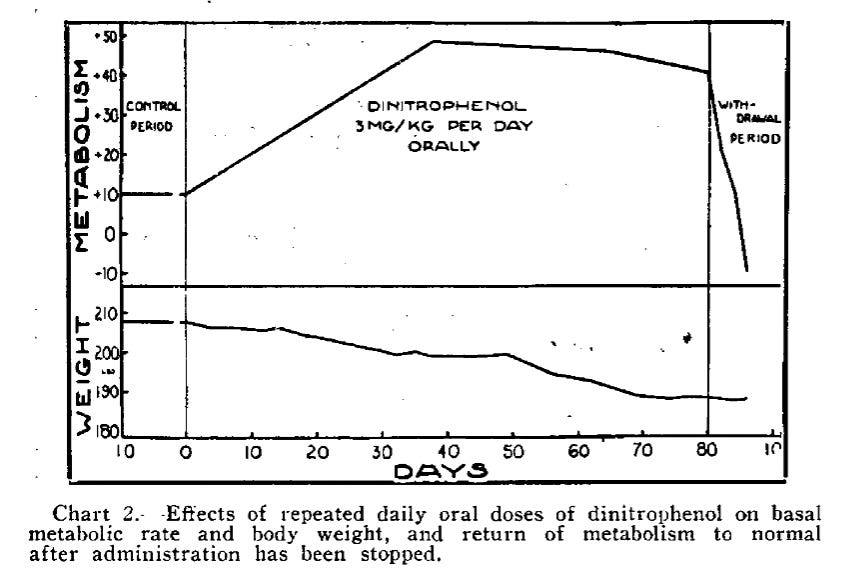
Perkins’ observation drew little attention for a decade or so, until a group of Stanford doctors dug it up and ran a clinical trial to test the effects of DNP on overweight participants. Their results, published in 1931, showed that daily administration of DNP led to a 40 percent increase in metabolic rate. Patients who were given DNP lost, on average, 0.9 kg of body weight per week; a number comparable to the effects of Ozempic today.
The results of this trial, and its subsequent coverage in the press, kicked off a surge in demand for DNP. One year from their publication, the Stanford doctors commented on the craze:
During the past year, the Stanford Clinics have supplied to physicians, or to patients on physicians' prescriptions, over 1,200,000 capsules of dinitrophenol… Since the usual daily dose is about 3 such capsules and the average duration of treatment about 3 months, this corresponds to 4,500 patients treated with the drug in a year. In addition, upward of 20 wholesale drug firms are marketing the compound, which suggests that a considerable population is being medicated. Probably at least 100,000 persons have been treated with the drug in this country alone.
Unlike hundreds of other purported weight loss drugs on the market at the time — from dangerous Jad Salts to Marmola (a “thyroid substance”) — DNP actually worked. But it came with a darker side: it quickly killed those who, like Georges, accidentally overdosed, as noted in a 1934 comment in Time magazine:
Drug companies have flooded the land with Dinitrophenol (usually under its right name, but one company calls it “Formula 761”) and many a druggist sells it without prescription. Last fortnight two cases of sudden death from overdoses of the drug were reported to the Journal of the American Medical Association, one of a young woman in Los Angeles who followed directions on the box and died violently within a week.
Still, there wasn’t much the U.S. government could do until the 1938 Federal Food, Drug, and Cosmetic Act gave authority to the FDA to regulate drugs. The agency promptly used its new powers to ban DNP. Other countries followed, with some classifying the chemical as a poison.
Today, few remember DNP. The molecule is little discussed outside of niche online bodybuilding communities, where some tout the substance as a quick way to lose body fat. Ozempic and the other GLP-1s now offer similar or better weight loss results for less risk. But the way DNP works to burn fat is different from GLP-1s and, if its dangerous side-effects could be tamed, the mechanisms could prove complementary. Such a partnership would work because, at a high level, there are two ways to target obesity: reducing energy intake through appetite modification (GLP-1s or gastric bypass) or by increasing metabolism (DNP).
The way that DNP increases metabolism is unusual for a drug molecule. Instead of modulating a particular protein or gene target, it modulates the function of an entire organelle: the mitochondria. As such, DNP belongs to a class of drugs known as “mitochondrial uncouplers.” To understand how uncouplers work, it is necessary to appreciate how mitochondria produce energy.
A mitochondria looks like a bunch of worms inside a bean. The “worms” are called cristae, protrusions of the inner membrane that separate the mitochondria into two compartments — the outer compartment surrounds the inner one like a rubber tire. Embedded inside the inner membrane are the proteins of the electron transport chain.
The electron transport chain proteins are nanoscale pumps powered by the breakdown of sugars and other macronutrients. Like water pumped uphill into a reservoir against gravity, these mitochondrial pumps push positively charged protons “uphill” against a concentration and voltage (an “electrochemical”) gradient into the outer compartment (the “intermembrane space”).

The imbalance of protons created by the pumps of the electron transport chain makes the interior of the mitochondria slightly negatively charged, which exerts an attractive, inwards-pulling force on the protons in the intermembrane space.
Normally, charged molecules like protons cannot pass through a lipid membrane without a channel.5 The mitochondria exploit the electrochemical gradient by providing only one route back across the inner membrane: through a channel in the turbine-like ATP synthase protein. Like water flowing down a hill, protons descending down the electrochemical gradient turn the crank of this turbine, which provides the energy required to snap ADP and phosphate together to form the body’s main energy storage molecule, ATP.
However, in the presence of an uncoupler, this process of energy capture is short-circuited. Considering how simple DNP is in structure, this disruption is remarkably effective. For a molecule to function as a mitochondrial uncoupler, it needs to have only a few properties.
First, it must be lipophilic; that is, the molecule must reside in uncharged lipid membranes and stay there. Second, it must be an acid, such that it can pick up and release protons. Most acids are charged, however, which means they cannot normally reside in lipid membranes. Mitochondrial uncouplers like DNP, though, have a particular arrangement of atoms that draws their charge inwards into the molecule, thereby masking it. In DNP, the NO2 groups serve this “withdrawing” function.
Once inside the mitochondrial inner membrane, the uncoupler moves back and forth like a riverboatman smuggling his cargo from one bank to another. Uncouplers equilibrate and destroy the proton gradient that feeds ATP synthase, preventing ATP generation.
Mitochondrial uncouplers heat the body in two ways: First, the non-productive passage of charged protons across the cell membrane forces potential energy to be dissipated as heat (somewhat like a space heater). And second, with the proton gradient continuously dissipated by the uncoupler, the cell faces an energy crisis as its ATP synthesis plummets. To compensate, the mitochondria ramp up the rate at which they burn through fat and glucose reserves in an attempt to make up the energy shortfall. This is like throwing fuel into a furnace riddled with holes; energy is released, but it cannot be productively harnessed, so it is released as heat. It is this increase in metabolism that is responsible for DNPs' weight-loss efficacy.
If the dose of the uncoupler is large enough, though, it kicks off an uncontrolled rise in body temperature. Like what happened to Georges, the body literally cooks itself from within.
And yet, there is evidence to suggest that the uncoupling mechanism can be productively harnessed.
For one, the body has evolved its own natural uncoupler: thermogenin or uncoupling protein 1 (UCP1). UCP1 sits in the inner membrane of mitochondria in “brown” fat tissue, and generates heat by inducing a controlled leak in the membrane that allows protons to flow through non-productively. This process, called non-shivering thermogenesis, is how newborns and hibernating mammals stay warm.
The discovery of a natural uncoupler has suggested a potential route to co-opt non-shivering thermogenesis to increase metabolic rate and induce weight loss in a similar, albeit safer, way than with DNP. Researchers have tried various strategies along these lines: pharmacological activation of existing brown adipose tissue, inducing the “browning” of white adipose tissue to create UCP1-expressing beige or “brite” fat cells, and direct transplantation of brown fat cells. Yet these research efforts are still in the early stages, and results have so far been inconsistent: the regulation of brown fat is complex and hard to control, and implanted brown fat is hampered by poor graft survival, inflammation, and the tendency to lose its thermogenic properties and "whiten" over time.
The challenges with brown fat activation approaches and the ongoing gold rush in obesity medicines have triggered some to take another look at the uncouplers.
While DNP is too dangerous to be used widely, there is a large chemical space of uncouplers to explore with varying therapeutic indices. Many molecules share the essential properties of lipophilicity, weak acidity, and the ability to withdraw electrons. Several commonly taken drugs have uncoupling activity, including aspirin (which partly explains its toxicity in overdoses) and niclosamide, an anti-tapeworm drug that kills parasites via an uncoupling mechanism, with only a mild effect on human metabolism. There are many more uncouplers with still stranger forms, including some truly bizarre-looking molecules like perfluorotriethylcarbinol and carboranes.
Other uncouplers are now being actively advanced as potential medicines: Controlled release formulations of DNP have shown promising results in animal models of diabetes and obesity, as have entirely new synthetic uncouplers. Rivus Pharmaceuticals raised over $100m to take their DNP prodrug, HU6, into clinical trials for obesity in 2022. Pharmacological induction of metabolic stress might prove useful outside of obesity, too. Cancer, increasingly recognized as a partially metabolic disorder, has also shown early hints of susceptibility to uncouplers.
Because there are so many forms an uncoupler can take, each with different effects on metabolism, it suggests that there may be a path for small molecules to induce fine-grained control of metabolism and mitochondrial energy expenditure — imagine the ability to tune your metabolic rate like one does a thermostat. A century or so on from the industrial accidents that killed workers like Georges, and the first weight loss craze, we may finally be learning how to tame these molecules.
Alex Telford is the founder of Convoke, a technology company building an AI workspace for biopharma commercial and medical teams, and a former consultant to the industry. In his spare time, he enjoys writing about biotechnology.
Cite: Telford, A. “The First Weight Loss Drugs.” Asimov Press (2025). https://doi.org/10.62211/82we-11pu
Lead image by Ella Watkins-Dulaney.
Picric acid was responsible for the Halifax Explosion.
Cresol had been France’s preferred desensitizer for picric-acid shell fillings before 1914, a shortage of cresol during the war led to its substitution by DNP. 40-60 mixture of DNP and TNP is the most used.
While trinitrotoluene (TNT) is far safer to store and handle than the TNP/DNP mixture (and TNT later supplanted TNP as the world’s preferred explosive), TNP remained the most common explosive used by France throughout the first World War: TNP manufacturing was already in place, and the raw inputs (particularly phenol#) were much easier to acquire — the supply of toluene required to manufacture TNT was principally controlled by Germany. French TNP production ramped from 0.5 metric tons per day in August of 1915 to a peak of 166 metric tons in mid-1917 (more than double the peak French TNT production).
Normally, protons cannot pass through a lipid membrane because, as charged molecules, protons are surrounded by a ‘hydration’ shell of polar water molecules that would first need to be shed — an energetically unfavorable step that makes the lipid membrane near impermeable.



Excellent article and discussion/description of the electron transport chain and mitochondrial uncouplers. Thanks Alex.
Why would uncouplers be good against cancer? Don’t cancer cells predominantly use anaerobic metabolism anyway?