Making the Centrifuge
The modern centrifuge was first designed for milkfat separation in the dairy industry. Today, it is ubiquitous in research laboratories. To whom do we owe its astonishing versatility?
The German Empire was founded in 1871, following Prussia’s victory in the Franco-Prussian War. Although a fledgling political nation, Germany was already renowned for its scientific genius. Many discoveries — from microscopy to organic chemistry — came from German scientists working in a period of increasing industrialization, economic growth, and military mobility. Among them were the Prandtl brothers, Antonin and Alexander, both trained as engineers in the applied sciences.
While a professor working in an experimental dairy station in Friesing, Upper Bavaria, Alexander would refine one of Antonin’s earlier designs into an invention worthy of the 1875 World Exhibition in Frankfurt am Main, where it would be showcased alongside synthetic dyes and early pharmaceuticals. The invention in question was a device that applied the Newtonian law of centrifugal motion — that rotating objects experience an outward force — to push denser materials outward (in this case, skimmed milk) while the lighter material (the cream) remained closer to the center.
Here were the beginnings of a scientific staple, the centrifuge.
Originally employed in the dairy industry to separate butterfat, the Prandtl brothers’ machine would undergo many revisions before becoming the ubiquitous industrial and laboratory tool we know today. With each refinement, the centrifuge became faster and more precise, and its applications broader. Higher speeds allowed scientists to exploit ever-slighter density variations, separating increasingly smaller particles — from tiny biological molecules like viruses or DNA to uranium isotopes sifted at the atomic level.
But greater speed presented its own challenges. Faster rotations made early centrifuges unstable, causing mechanical stress. Overheating due to friction often damaged samples. In response, engineers had to figure out how to reinforce rotors and install automatic balancing and refrigeration systems.
And so, as it moved from milk to minute particles, the centrifuge followed a similar trajectory to that of other scientific instruments, adapting, through incremental and clever innovation, into a fundamental research tool.
The Centrifuge in Industry
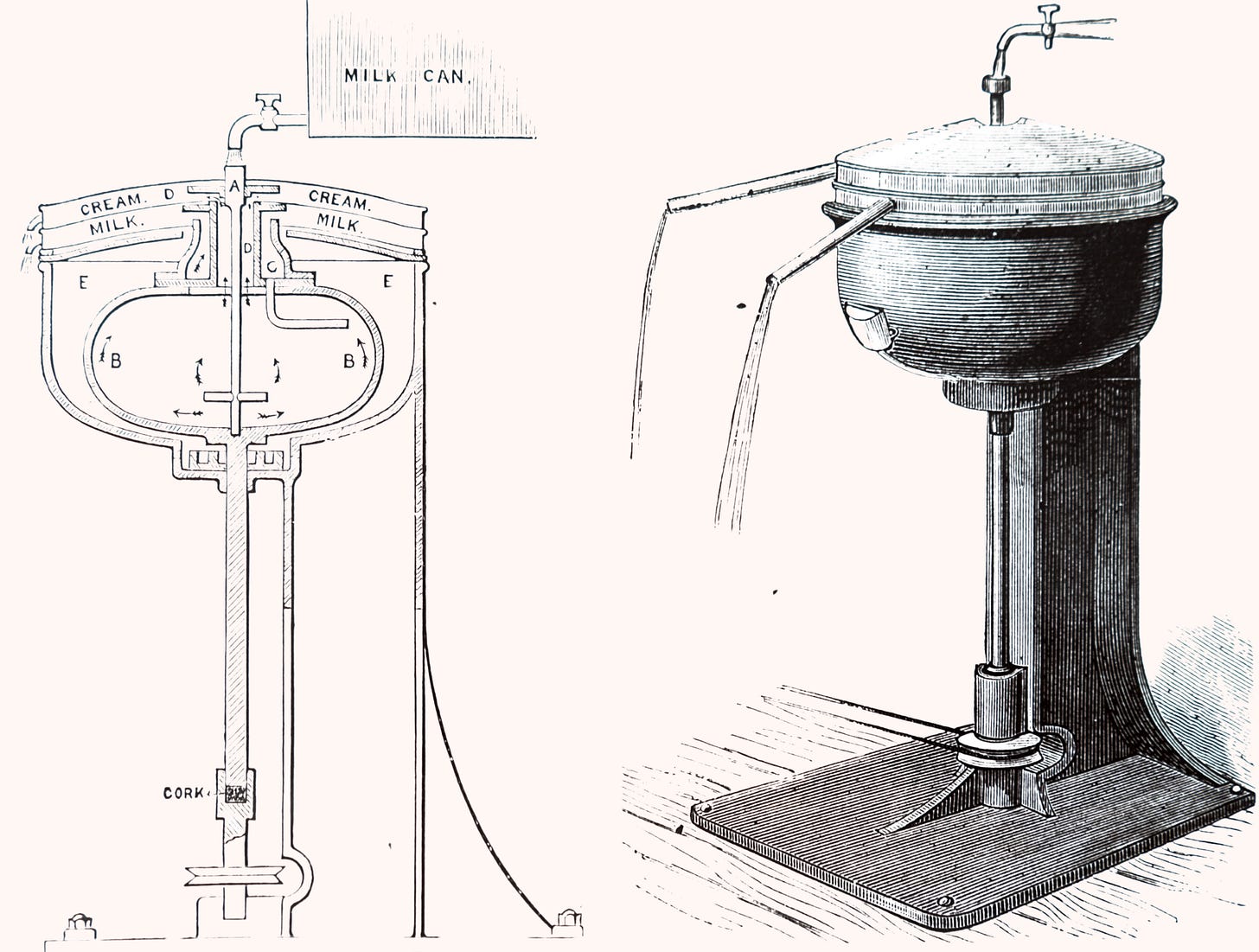
As early centrifuges were not the stable, temperature-controlled devices we know today, they were first used to separate comparatively large molecules, such as those in whole milk. On July 3, 1878, just three years after Alexander Prandtl’s centrifuge caused such a stir at the Frankfurt World Exhibition, Swedish engineer Gustaf de Laval patented the first continuous-running centrifugal milk-cream separator. Imbued with patriotism, de Laval declared: “I will show that centrifugal force will act in Sweden as well as in Germany.”
De Laval’s drum design had a diameter of 60 cm and a rotational speed of 3,000 rpm. Milk entered the rotating drum through an inlet pipe at its center and was forced outward through channels at its base. As the drum spun, the heavier skimmed milk moved toward the edge while the lighter, less dense cream collected toward the center. The skimmed milk on the outside was channeled up and out of the machine via the lower outlet, while the cream was expelled through the upper outlet. New supplies of whole milk entered continuously through the inlet, displacing the separated liquids in the drum through individual channels and forcing them upward into collecting covers at the top.
Even after being granted his patent, de Laval continued to make improvements, such as decreasing the diameter of the drum — now referred to as the bowl — to increase rotational speeds. One year later, de Laval’s bowl diameter had decreased to 29 cm, and the speed had increased to 5,500 rpm. In 1883, de Laval and his partner, engineer Oscar Lamm, founded AB Separators and, by 1886, the duo had designed an easily mass-produced centrifuge capable of processing 400 liters of milk per hour.
Around the turn of the 20th century, electric motors became available, and before long, centrifuges became key devices in an even wider range of industries. In laundries and textile processing, spinning drums helped force liquid out of fabric before drying. In the sugar industry, they separated sugar crystals from molasses, and in honey production, they aided in separating honey from the combs without destroying them, allowing for their reuse.
While it was evident that centrifugal motion was more efficient than traditional gravity-based separation, these first industrial centrifuges still needed to become faster and more precise. In honey extraction, for example, unless the delicate combs were carefully wired in place, the centrifugal force could crush the fragile wax. The separation of smaller biological particles required an even more delicate balancing of the centrifuge’s rotors as even the slightest vibration could cause the separated materials to remix.
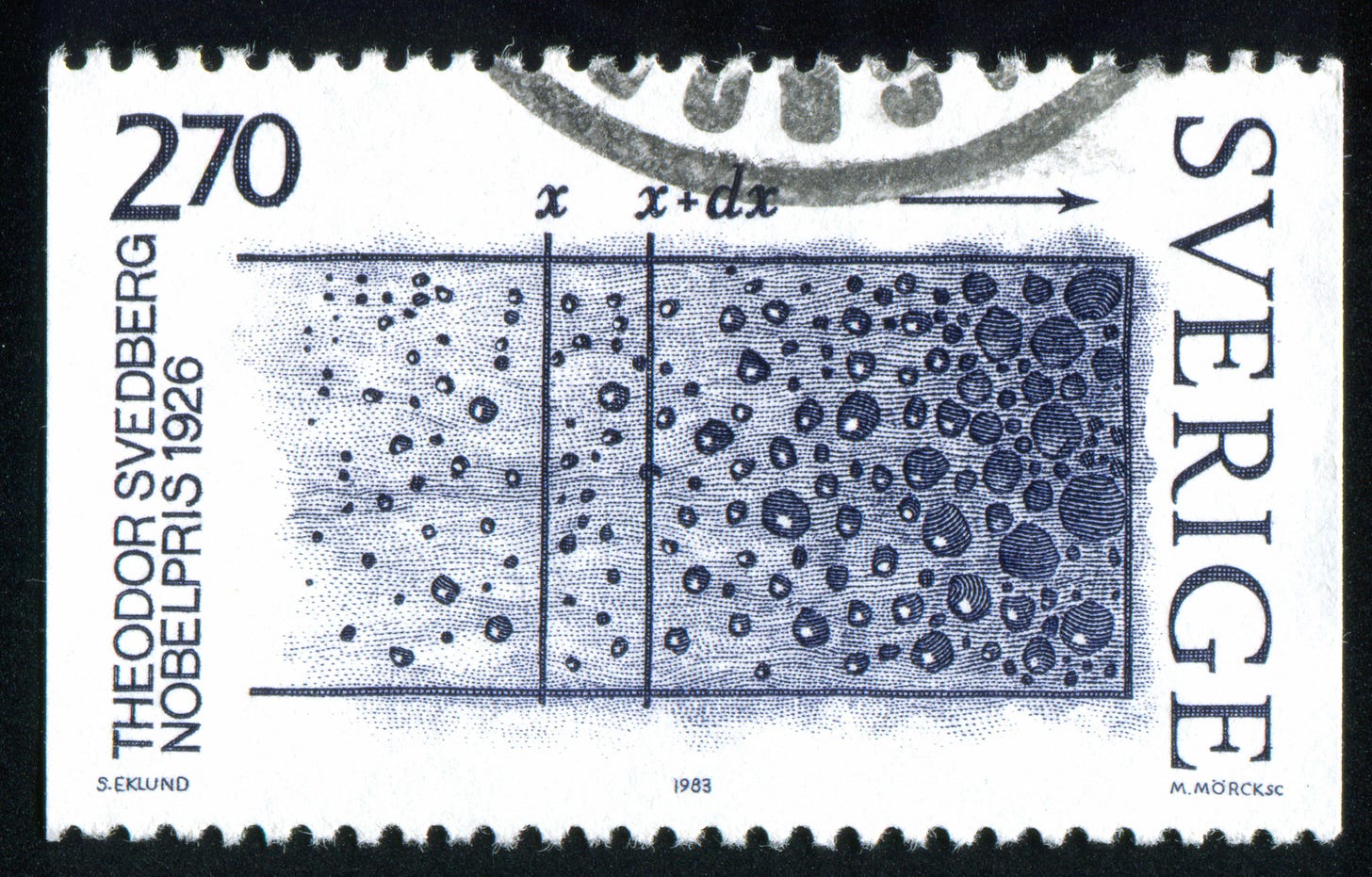
Within a generation, the Swedish physical chemist and eventual Nobel Prize winner, Theodor Svedberg, addressed these issues, introducing a much faster centrifuge to aid in his investigation of colloidal composition. Colloids are water-based solutions of tiny particles too small to see but still larger than individual molecules.
Well-educated in both chemistry and physics, Svedberg reasoned that his colloid solutions consisted of particles of different sizes.1 He believed that he could determine the sizes of these particles by using Stokes’ law to measure how much light they blocked as they settled out of suspension. However, gravity alone would not suffice to separate the finer particles, nor would extant centrifuges. Svedberg needed a device that spun at much higher speeds than their current capacity of 3,000 to 5,500 rpm.
In 1923, while crossing the North Atlantic to instruct students on colloid science at the University of Wisconsin–Madison, Svedberg busied himself by sketching designs for an apparatus that would meet his needs.
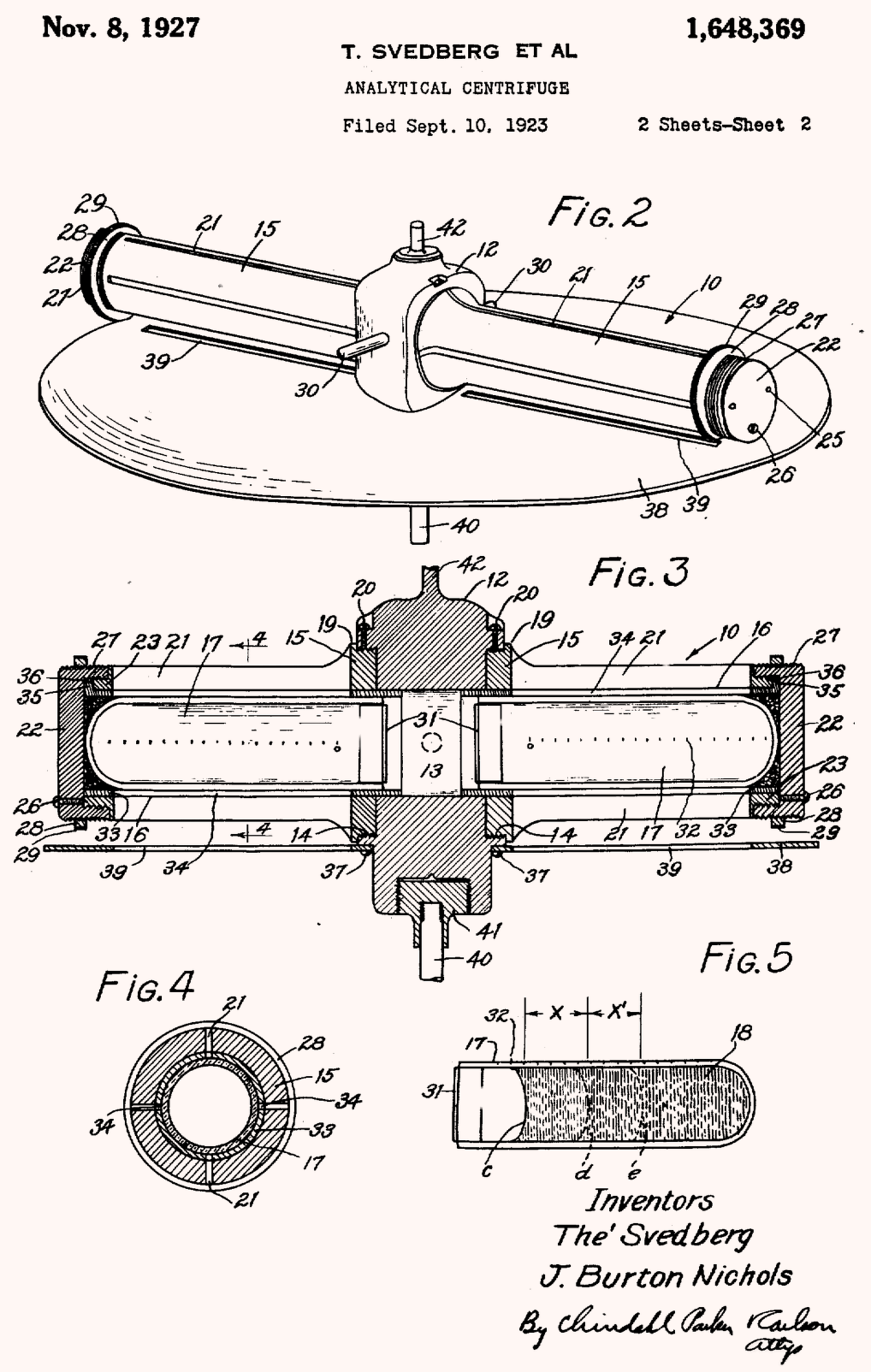
He drafted his device mounted inside a steel box to control both temperature and air currents. The centrifuge would have an optical monitoring system to observe particles as they settled. The rotor would have two arms with a disk slotted so that light could only pass through when the sample was in correct alignment and a camera for imaging. An electric motor would spin the machine at an unheard of 20,000 rpm. Upon arriving at the campus, Svedberg set to work with physicists at the University of Wisconsin to create a prototype. He called his invention the ultracentrifuge.2
Built over a single spring term, Svedberg’s new ultracentrifuge could force even the lightest molecules out of suspension. The centrifuge’s camera allowed Svedberg to analyze the photos and measure how fast molecules fell. With such data, he could then determine the molecular weight of each molecule.
On his trip back across the Atlantic, Svedberg continued tinkering with the prototype he had built in Madison. He focused on improving rotor balance and creating stronger centrifugal forces to allow for more precise measurements of differences in particle size, shape, and density.
Once back in Sweden, Svedberg and his colleagues worked to remove the dangerous kinks that remained. The rotational forces on the rotors were intense, and malfunctions could spray metal shrapnel across the lab. A new rotor was crafted from high-tensile steel and carefully shaped on a lathe to maximize its strength. The new design placed the rotors inside heavy, steel safety blocks that also helped stabilize temperature, and the system operated in a low-pressure hydrogen atmosphere to manage heat. The result was a faster, stronger, and safer machine capable of spinning large molecules at centrifugal forces up to about 106 gravity or approximately 100,000 rpm.3
Svedberg also collaborated with engineers at Ljungström Steamturbine Co. to develop an oil-turbine drive system that improved lubrication and reduced vibrations, allowing the centrifuge to operate for hours on-end.
However, it proved challenging to create a viewing compartment to hold the sample — the centrifuge cell — that would not crack under stress. The early designs used quartz windows, but they frequently failed. So, while his new centrifuge was faster, the materials available at the time limited its maximum speed. Later versions benefited from synthetic sapphire windows, which reduced optical distortion and breakage.4
Prior to Svedberg's work, the molecular structure of proteins was unknown. Many scientists believed they were loose, random clusters of molecules or amino acids weakly held together, referred to as colloidal particles. Others envisioned them as large single molecules, but no chemical analytical tools yet existed to confirm this.
Svedberg’s ultracentrifuge demonstrated that proteins are discrete homogenous macromolecules with specific structures and molecular weights, rather than loose aggregates or colloids. In 1926, Svedberg was awarded the Nobel Prize in Chemistry for his work in colloids and for developing the ultracentrifuge.5
A further challenge remained, however. While analytical ultracentrifugation allowed researchers to observe the size, weight, shape, and purity of molecules, these samples could only be analyzed in real time. Individual components could not be collected because the layers would remix once the rotation stopped. While this didn’t impede Svedberg’s work, many avenues of scientific research required collecting separated materials for further study.
Émile Henriot, a French chemist and physicist based in Belgium, worked with scientist E. Huguenard to enable the leap to a centrifuge that allowed for the creation of preparative samples. In the 1920s, Henriot and Huguenard improved Svedberg’s design by adding compressed air jets that drove the rotor with less friction, allowing for high speeds without excessive heat. Their refinements also made it possible to spin tubes in different positions — upright, tilted, or at an angle — instead of lying flat on their sides. This allowed particles to settle at the bottom of the collection tube, forming a compact pellet. Once the spinning stopped, the tightly packed pellet stayed in place, preventing the remixing of the materials and permitting the collection of different layers.

From Industry to Isotopes
Also in the 1930s, at the University of Virginia, Jesse Wakefield Beams, an American physicist, applied Henriot and Huguenard’s design to his study of even smaller particles, individual chlorine isotopes, which necessitated separating out atoms of the same element that have the same number of protons but different numbers of neutrons in their nuclei. Beams recognized that rotation speed had to increase to provide the necessary force to separate such small particles. He also understood that the limiting factor for speed would be friction caused by atmospheric resistance.
His initial ultracentrifuge reached rotational speeds of 240,000 rpm. While unprecedented, Beams suspected that if he could eliminate rotational drag, it might be possible to reach speeds of a million or more revolutions per second. Other researchers had tried to do the same, albeit without success, because high-speed rotation created heat that burned out rotors and caused convective currents that remixed separated chlorine gases. Beams, however, had the clever idea to place the centrifuge in a vacuum, eliminating air resistance and reducing friction.
His vacuum ultracentrifuge worked marvels, going from thousands of rotations per second to millions, allowing the separation of molecules with only minute differences in mass. Before long, Beams turned his attention from chlorine to uranium atoms.
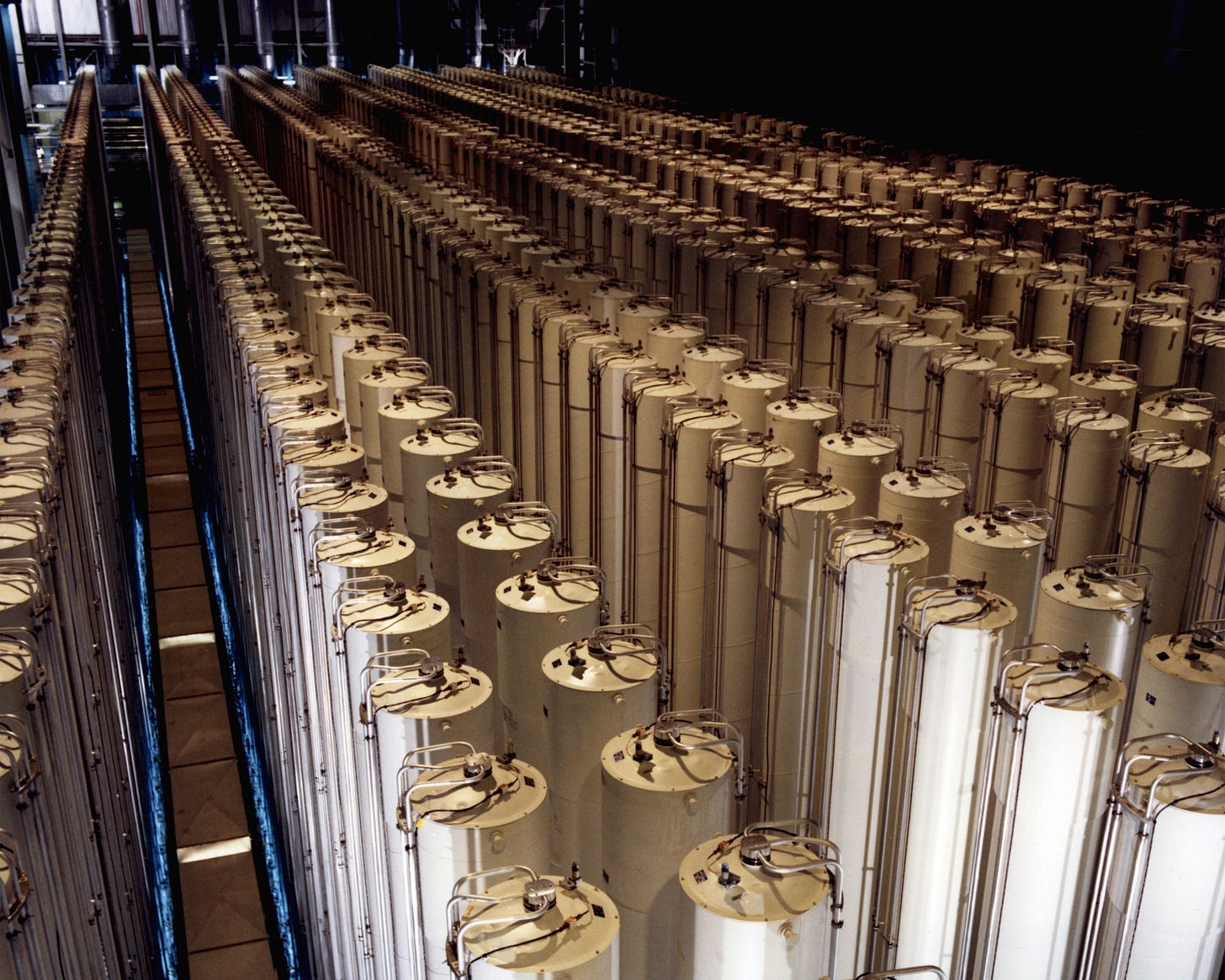
With the onset of World War II, Beams’s skillset made him an ideal recruit for the Manhattan Project. Over a four-year span, his ultracentrifuge was used for separating the lighter uranium isotope U-235, the less abundant but only naturally occurring uranium isotope capable of sustaining a nuclear chain reaction, from heavier ones — the concept behind uranium enrichment.6
However, it became apparent that Beams’s centrifuge still presented significant mechanical challenges. Even with its vacuum design, its high rotational speeds continued to produce severe vibrations that often led to equipment failure before sufficient quantities of the uranium isotope could be isolated. Ultimately, the project leads turned to the diffusion method instead, in which uranium hexafluoride gas was forced through a series of porous membranes. The lighter U-235 diffused slightly faster than the heavier U-237, gradually increasing its concentration. While the process presented challenges, as the filter had to be strong enough to withstand the pressure of the gas and vacuum sealed to prevent contamination and clogging, its output surpassed that of the current iteration of the centrifuge. Not long after, Beams’s time with the Manhattan Project ended.
Meanwhile, an Austrian-born Russian prisoner of war, Gernot Zippe, was being held in a secret Soviet research facility and tasked with overcoming the very limitations with the centrifuge that had ended Jesse Beams’s venture into the Manhattan Project.
Educated in Vienna as a doctor of mechanical engineering and physics, Zippe’s passion was aeronautical engineering. During World War II, he served as a civilian flight instructor for the German Luftwaffe — the aerial warfare branch of the German military. As the war was ending, Zippe was captured by the Soviet Army in Prague and brought to Stalingrad.
Soviet intelligence quickly learned of Zippe’s technical background and moved him to Sukkumi, on the Black Sea. There, Russians had established a secret research institute staffed with other scientist POWs working on isotope separation for creating weapon-grade uranium. Here, Zippe and his fellow Austrian POW, Max Steenbeck, corrected the issues related to stability and reliability that had plagued Beams’s efforts to develop a centrifuge for the Manhattan Project.
They designed the rotor to spin on the tip of a tiny needle, similar to a toy top, which reduced friction. By using loose bearings, they let the centrifuge self-adjust instead of trying to force it into balance. Counterintuitively, this greater freedom stopped it from shaking,7 allowing them to construct a reliable and stable gas centrifuge capable of large-scale uranium enrichment.
After being released from the Soviet facility in 1956, Zippe remained in Germany, where he became a consultant for centrifuge technology. At the 1957 Proceedings of the International Symposium on Isotope Separation held in Amsterdam, Zippe realized that the technology he had developed alongside Steenbeck while in captivity outpaced that in the rest of the world. Others noticed, too, and in 1958, Beams convinced Zippe to join him in the United States to continue working on centrifuge technology.8
Although the Soviet Government had confiscated his notes upon his release, Zippe and Beams were able not only to recreate his centrifuge but improve it. By switching the rotor material from aluminum to a superhard alloy called maraging steel,9 they enabled the centrifuge to spin faster without shaking itself to pieces. Additionally, they elongated the rotors, increasing their flexibility. Today’s Zippe-type centrifuges, used predominantly for nuclear fuel, are based on this design. Zippe eventually left the University of Virginia and returned to Europe as a consultant for URENCO — a British, German, and Dutch nuclear fuel consortium with plants in England, Germany, the Netherlands, and the United States that all used Zippe’s centrifuge enrichment technology.10
The Contemporary Centrifuge
While work on the large-scale Zippe-type centrifuge for uranium enrichment was advancing in Virginia, across the Atlantic in Hamburg, centrifuges were being miniaturized. Larger centrifuges were critical to industrial processing and nuclear isotope separation efforts, but in the life sciences and biomedicine, microcentrifuges were needed, able to produce finer separation and handle smaller volumes of biological samples with precision.
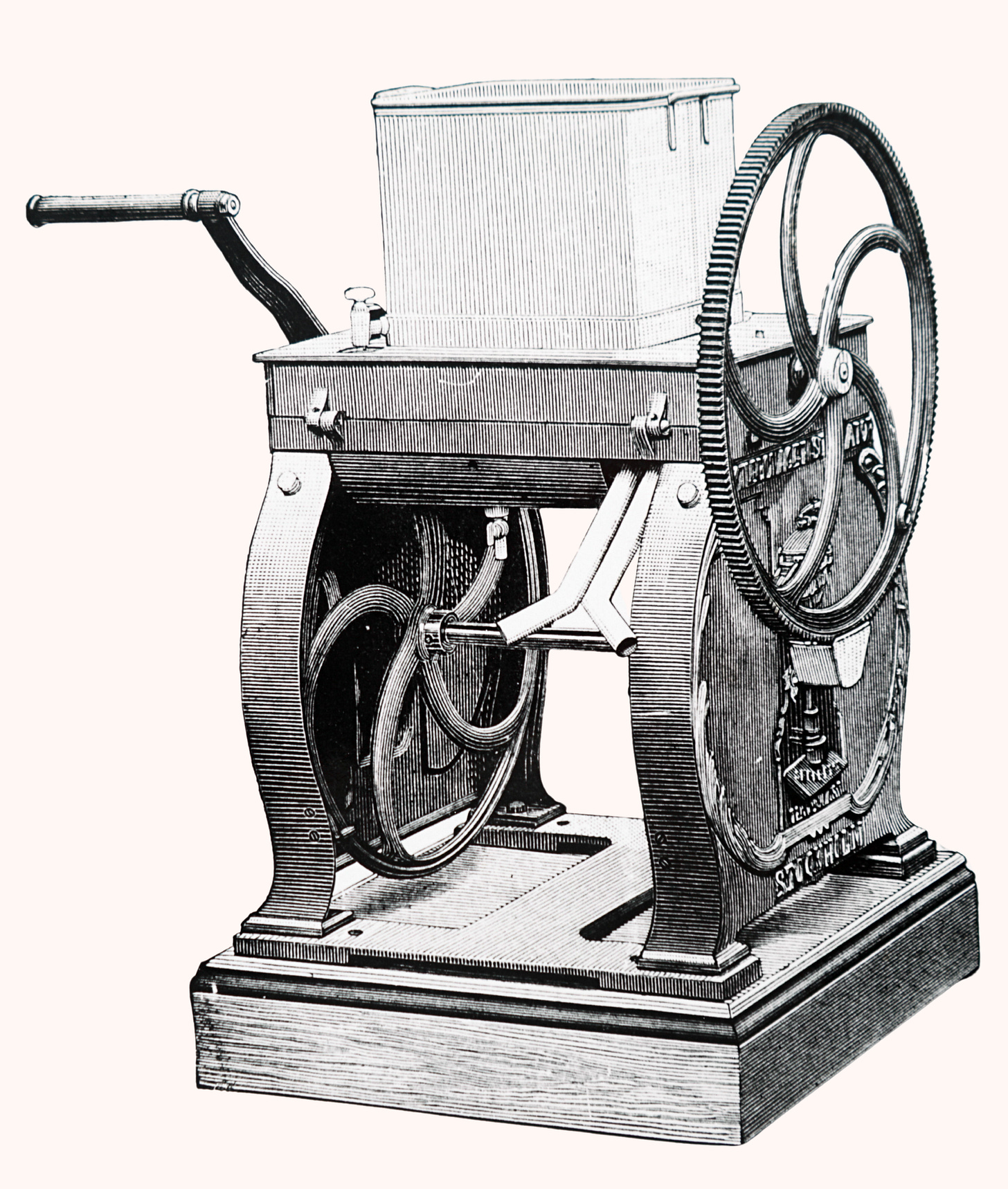
Of course, as early as the 19th century, biologists had already been modifying existing centrifuges for their work. Swiss biologist and physician Friedrich Miescher used a hand-cranked version similar to that of the Prandtl brothers to isolate the constituents of the cellular nucleus. In 1869, this centrifuge allowed Miescher to identify a new substance rich in phosphorus along with the usual proteins and lipids. Because the substance came from the nucleus, Miescher named it nuclein — today known as nucleic acid (or DNA).

In the 1950s, the biochemist Christian de Duve would use a centrifuge for the process of cell fractionation, aiding his identification of lysosomes and peroxisomes, which have proved central in our understanding of cellular digestion, cellular metabolism, and detoxification.
Modifications to the centrifuge continued, and in 1962, the leading life science product developer Eppendorf developed the first laboratory microcentrifuge able to handle smaller samples, as small as 2 mL or less, at exceptionally high speeds. This allowed for the isolation, purification, and study of minute amounts of DNA, RNA, proteins, and cells — tasks ill-suited to the larger centrifuges.
The next leap forward came in 1976 when the German laboratory equipment and technology company Andreas Hettich introduced the world to the first microprocessor-controlled centrifuge at ACHEMA, a leading trade show for the chemical, pharmaceutical, and biotech industries. This occurred only five years after the first microprocessors were released to the public. These microprocessor-controlled centrifuges let researchers set exact RPM (revolutions per minute), enabling consistency across experiments and reducing human error. Automatic programming allows these centrifuges to store multiple protocols and enables pre-programmed cycles, making clinical diagnostics more reliable, efficient, and reproducible. Such programming has been similarly useful in genomics and genetics research, where robotic centrifuges streamline steps and procedures.
As innovations broaden their applications further still, centrifuges have even moved into space. A special Hettich-designed and created centrifuge is currently used on the International Space Station (ISS) in the Destiny Laboratory, studying how space travel affects human physiology. Of special interest are immune dysregulation, muscle loss, and the extent to which astronauts' cells react to the presence or absence of gravity at a molecular level. The centrifuge provides an opportunity to observe these changes and potentially pave the way for longer space journeys.
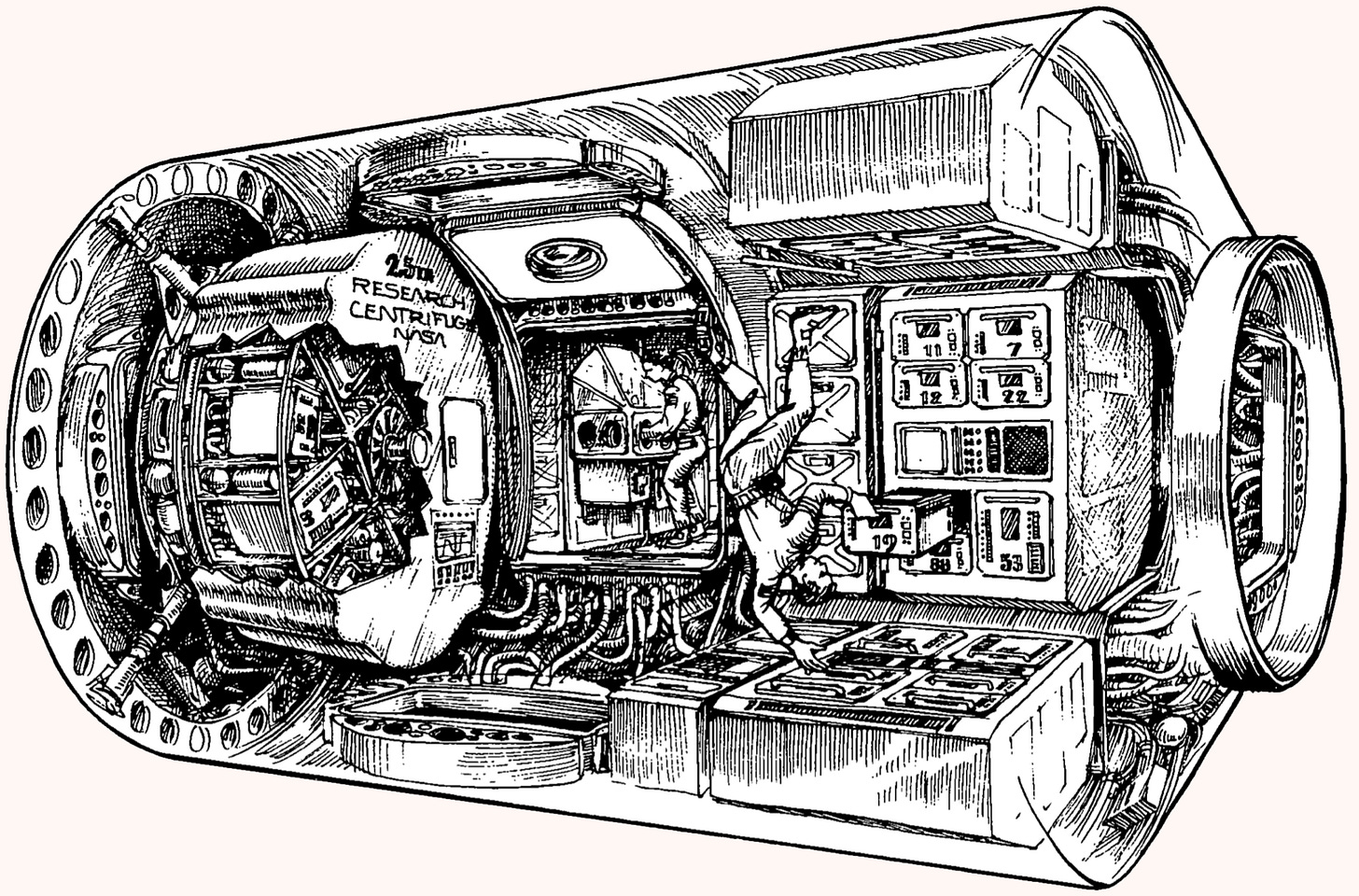
Back on earth, at NASA’s Ames Research Center in California’s Silicon Valley, centrifuges recreate high-gravity conditions to train pilots and astronauts for the extreme forces they will experience in flight.
The Prandtl brothers’ first centrifuge has proven exquisitely flexible. While the general principle of spinning substances to separate them has not changed, targeted modifications by practical scientists such as Svedberg, Henriot and Huguenot, Beams, and Zippe have made the device key to a wide range of human endeavors.
Doctors diagnose diseases and separate blood into components for testing and treatment. Pharmaceutical and biotech researchers produce vaccines, extract DNA and RNA, separate nanoparticles, and prepare samples. The energy industry separates oil, water, and solids during drilling and refining and in nuclear power production. Environmental engineers harness centrifuges to treat wastewater by removing solids from liquids.
In his 1974 Nobel Prize acceptance speech, Christian de Duve expresses his gratitude to this ubiquitous tool. Referencing a Swedish tale by Selma Lagerlöf, the first woman to win the Nobel Prize in Literature, in which a boy crosses the country on swanback, de Duve says: “I too have made a wonderful journey, using … an unconventional mode of travel. For the last 25 years, I have roamed through living cells, but with the help of a centrifuge rather than of a microscope.”
Roberta McLain is a freelance science writer and high school science teacher based north of Boston with a deep-seated passion for neuroscience, evolution, anatomy and physiology, the allure of zoology, and all things life sciences. She has master's degrees in biology and science writing. Her work has also appeared in publications such as Scientific American, The Science Writer, Science News Explores, and Live Science.
Cite: McLain, R. “Making the Centrifuge.” Asimov Press (2025). https://doi.org/10.62211/73rf-55kd
Lead image by Ella Watkins-Dulaney.
The young man was so bright and handworking that when he arrived at Uppsala University in 1904, he was exempted from practicals and earned his bachelor’s degree in about a year.
In naming it the “ultracentrifuge,” Svedberg was both emphasizing the speed of the centrifuge and paying tribute to Richard Zsigmondy, the inventor of the ultramicroscope whose pioneering work on colloids impressed him. Svedberg saw his centrifuge as a complement to the ultramicroscope — an “ultra‐” device that allowed people to observe even smaller particles by subjecting them to greater centrifugal forces.
With centrifugal forces of 10⁶ g and a rotor radius of 0.1 meters, the centrifuge would spin at about 94,000 rpm. The exact speed would depend on the radius of the centrifuge.
Although technically a crystal as opposed to true glass, the term “sapphire glass” is used because of its transparency and its use as a glass substitute. While Svedberg was redesigning his ultracentrifuge in 1926, German scientist Spyro Kyropoulos was developing a method for growing large, high-purity single sapphire crystals that would prove ideal for optical windows and scientific instruments.
Organic materials, like blood and proteins, were hard to study because they were found in mixtures. The ultracentrifuge allowed the materials to be separated, so particles with the same densities formed distinct bands. Scientists could calculate the molecular weight of the materials if they knew the velocity necessary to separate them and where they ended in the tube.
Natural uranium contains only 0.7 percent U-235; the majority is U-238. Consequently, the race was on to speed up the separation of the lighter uranium from the heavier isotopes — a process referred to as uranium enrichment.
This uses a similar principle to various seismic retrofitting architecture, such as base isolation systems, which allow the building to move around on its foundation to absorb seismic energy and reduce how much ground motion transfers to the structure.
He may not have needed much convincing. In Zippe’s own words, “The Russian influence in Europe, especially in Germany, is so dangerous … you have to transfer me to the United States … knowing the capability to make atomic weapons, I want to give the technology exclusively to the Americans.”
Maraging steel is known for its superior strength and toughness; the name “maraging” comes from the martensitic aging process, the heat treatment which strengthens the steel.