The World’s Most Common Surgery
In 4,000 years, cataract surgery went from a crude procedure involving thorn instruments to a 20-minute operation with a 95 percent clinical success rate. The next step is broadening access.
Today we launch Issue 08 of Asimov Press. Read our full Editors’ Note and preview upcoming articles on our website. Thanks for reading!
Sight has never merely meant the detection of light. In almost every culture, the eye serves as a sacred symbol: the all-seeing Eye of Horus in Egypt, the divine eye of Odin in Norse mythology, and the third eye of inner knowledge in Hinduism. Like the heart, this organ carries mythological weight and has been intimately linked to the soul.
But unlike the heart, vaulted away behind layers of tissue and bone, eyes are exposed, leaving them vulnerable to debris and external damage. Also, unlike the heart, the lens of the human eye has no blood vessels, no nerves, and no interaction with the immune system, meaning it cannot absorb medicines, respond to immune signals, or recover from structural injury.1
Throughout much of history, then, when an eye was damaged, there was little that could be done to heal it. And even today, ophthalmologists like myself struggle to address the idiosyncrasies of this organ when treating ocular and vision disorders.
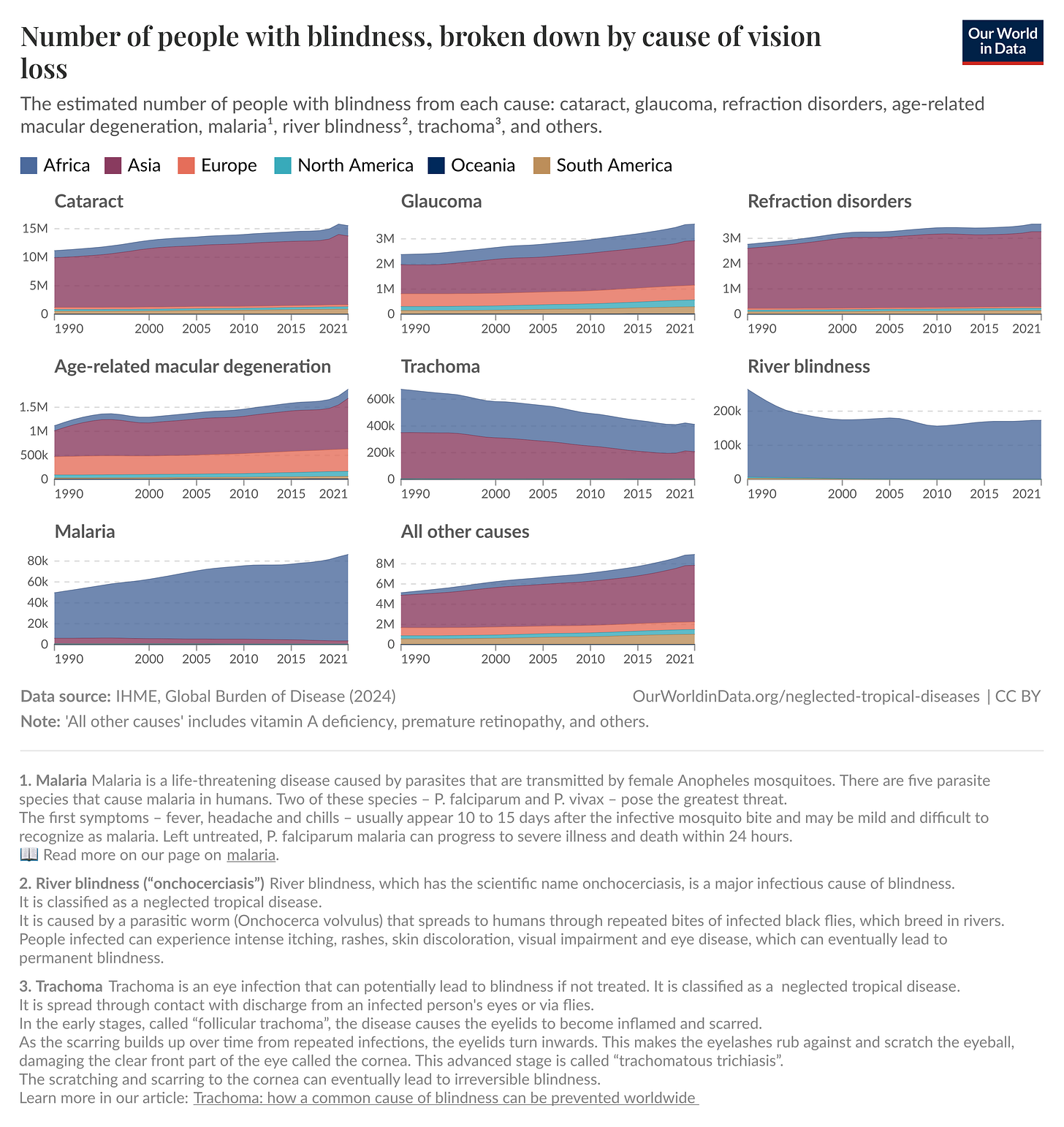
And such disorders are rampant: In 2020, an estimated 596 million people had distance vision impairment worldwide, of whom 43 million were blind. While the cause for blindness varies — cataracts (17.01 million cases), glaucoma (3.61 million cases), trachoma2 (1.9 million cases), age-related degeneration (1.85 million cases), and more — these diseases have enormous implications for health and economic development when taken together. Some of these problems, like age-related macular degeneration, remain difficult to treat, but others, such as cataracts, have ready and cost-effective interventions.

Cataracts are caused by the clouding of the lens due to protein accumulation and denaturation. This buildup must be removed, or it can lead to blindness.3 Because this protein denaturing commonly occurs with aging, cataract surgery is the most frequently performed procedure in all of modern medicine, with more than 20 million operations carried out annually across the globe (surpassing cardiac stents, hip replacements, and appendectomies.) Compared to other surgical interventions, cataract surgery is also fairly affordable, ranging from $6,000 in a high-income country like the U.S. to as low as $150 in a country like Tanzania.
Despite cataract surgery’s prevalence and affordability, however, vision loss from cataracts remains the leading cause of blindness worldwide, especially in low-income countries. So while it is tempting to champion cataract surgery as one of medicine’s most enduring and continually refined surgical interventions (today the surgery takes around 20 minutes and has a success rate above 95 percent), it is not yet a wholesale success story. After all, progress isn’t only about advancement in technique but also broader access — especially when the costs of providing it are marginal.
Cataracts in the Ancient World
The word cataract originates from the Latin cataracta and the Ancient Greek katarrhaktēs, both meaning “waterfall.” This imagery likely stemmed from the observation that milky lens opacities resembled cascading water. In classical Latin, cataracta could also denote a “portcullis,” a heavy gate that blocks passage; apt symbolism for the visual obstruction caused by the condition.
The first explicit mention of cataracts as a distinct and treatable pathology comes from the Sushruta Samhita, composed circa 600 BCE. It was written by the renowned Indian physician Maharshi Sushruta and contains the first comprehensive surgical explanation of the antecedent to cataract treatment, a procedure known as “couching.”
Couching entailed pushing the clouded lens (the cataract) into the vitreous chamber, or back portion of the eye, using a curved needle. Crucially, this procedure merely shifted the cataract away from the pupil to allow light into the eye, temporarily restoring limited sight without actually removing the cataract. Patients were often left with poor focus or blurred vision as a result.

Although the couching procedure described by Sushruta was rudimentary compared to modern cataract surgery, it was meticulous for its time. The procedure began with the patient seated upright, gazing steadily at the tip of the surgeon’s nose, while the surgeon positioned themself opposite the affected eye. The surgeon would identify a specific entry point on the sclera, the white part of the eye, carefully avoiding any visible blood vessels. Holding a rod-like instrument (sometimes made from thorns or metal) firmly between the thumb, middle, and index fingers, they would then puncture the sclera just beside the clear front surface of the eye known as the cornea. A subtle sound and fluid release signaled successful entry into the eye chamber.
The surgeon then gently pushed or scraped the lens to move it out of the line of sight. The patient was asked to close the nostril opposite the treated eye and blow through the other, a technique believed to help expel the “phlegm” (kapha) from the eye.
Sushruta stressed the importance of hygiene, timing, and patient preparation, recommending the surgery only in favorable seasons, after fasting. Afterward, the eye was washed with breast milk, warmed with herbal preparations, and bandaged with clarified butter (ghee). Patients were advised complete rest and cautioned against coughing or sneezing.
Despite its limitations, couching became the mainstay of cataract treatment for centuries across India, Persia, Europe, and the Islamic world. Sushruta’s work left a lasting impact on the field, with careful surgical methods rooted in an understanding of the eye’s anatomy.
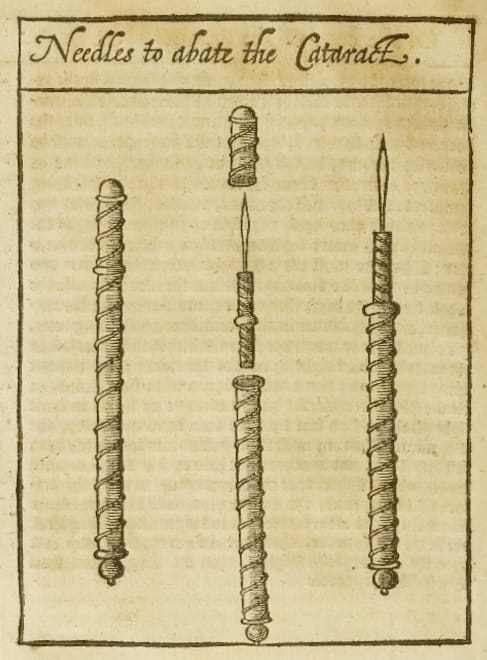
In the Hellenistic and Roman world, the first references to the treatment of cataracts can be found in 29 B.C. in De Medicinae, the work of the encyclopedist Aulus Cornelius Celsus. Within, Celsus described a couching procedure, in which “… the needle … must be thrust … in the middle part betwixt the black of the eye (the limbus) and the external angle (the lateral canthus) [until]… it comes into a void space (inani loco).” While evidence of sharp instruments from excavations across Babylonia, Greece, and Egypt suggests the widespread use of couching, Greco-Roman surgeons did not so much advance the treatment as proliferate it, borrowing from methods recounted by travelers returning to the Mediterranean from India.
The next true surgical advance came from the Islamic world, during its “Golden Age.” During a period when much of medieval Europe was experiencing intellectual stagnation, marked by limited access to classical texts and the decline of formal medical institutions, the Islamic world flourished as a center of scientific advancement. Between the 9th and 14th centuries, cities like Baghdad, Cairo, and Córdoba boasted hospitals (bimaristans), medical schools, and observatories whose practices allowed ophthalmology to grow into a refined specialty.
A key figure from this period was the physician Ammar ibn Ali al-Mawsili, who was born in Mosul but did most of his work in Egypt. In the 10th century, Al-Mawsili wrote a book on ophthalmology, Muntakhabfi ‘ilaj al-’ayn (The Select Work on the Treatment of the Eye), in which he discussed various ocular pathologies and promoted an alternative to couching. His technique made use of a hollow needle of his own design, which he used to suck out the cataract.
His innovation marked a distinct conceptual evolution: the lens was no longer a part of the eye to be pushed aside, but a pathological entity requiring removal.
Extraction Techniques
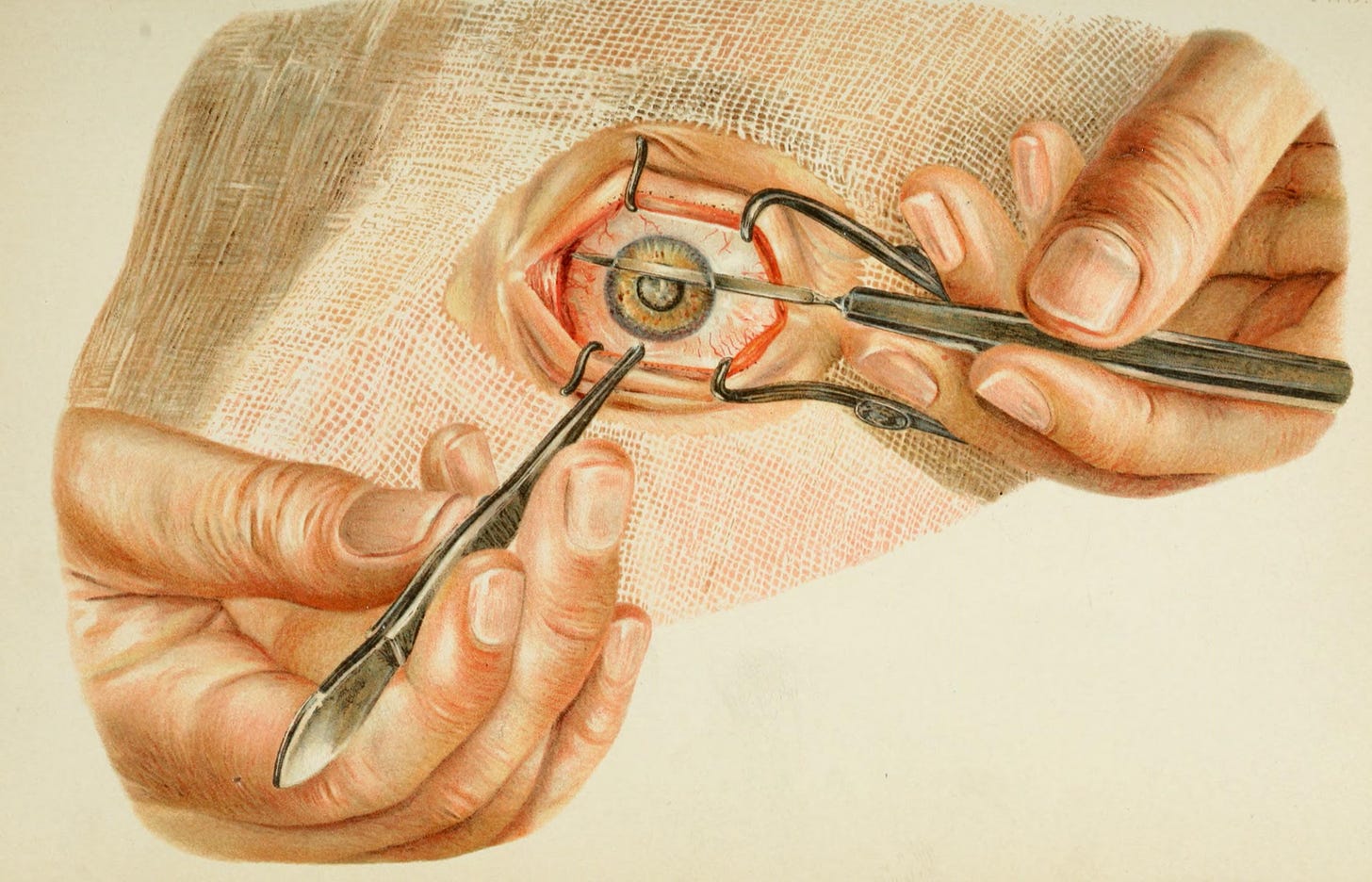
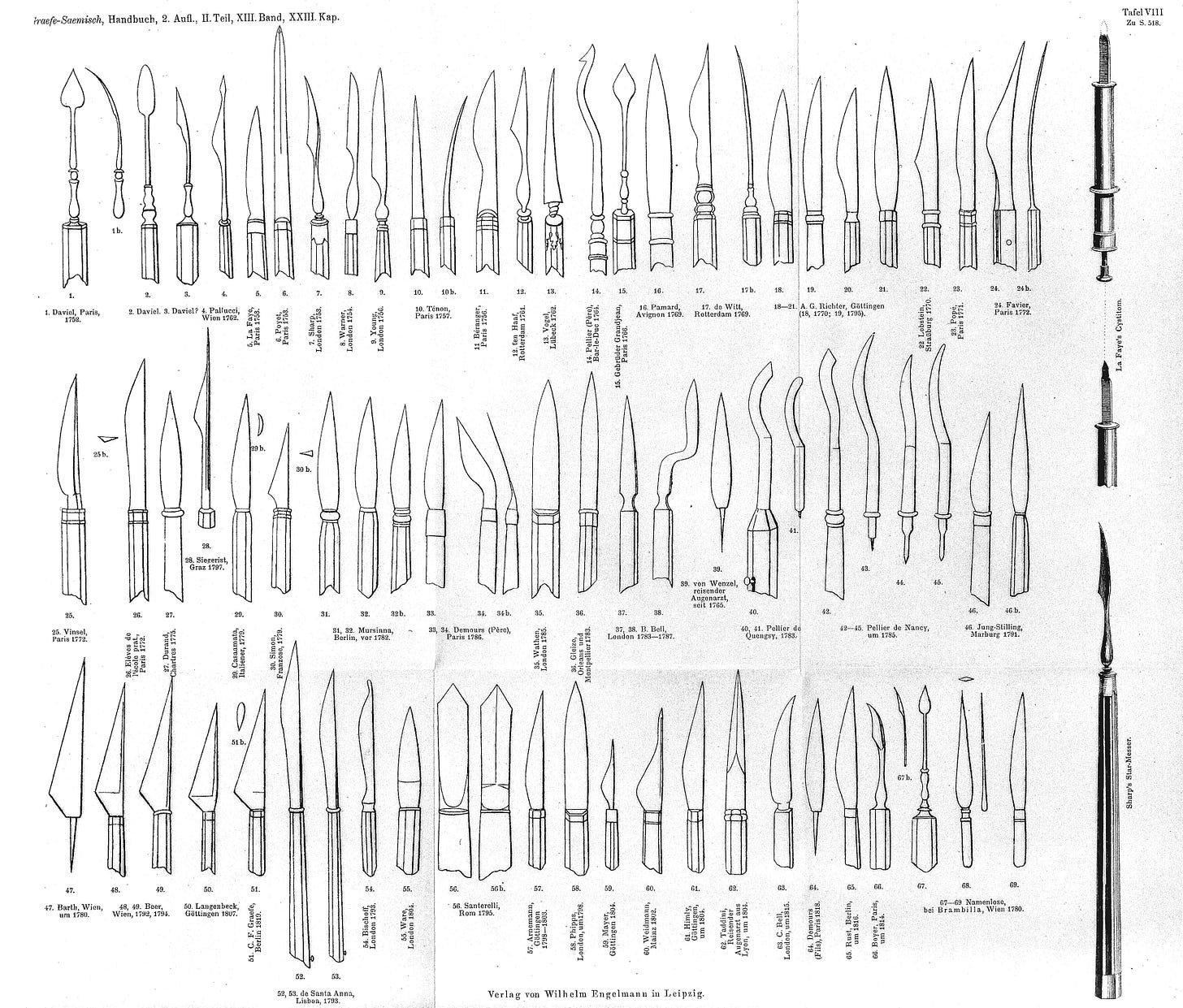
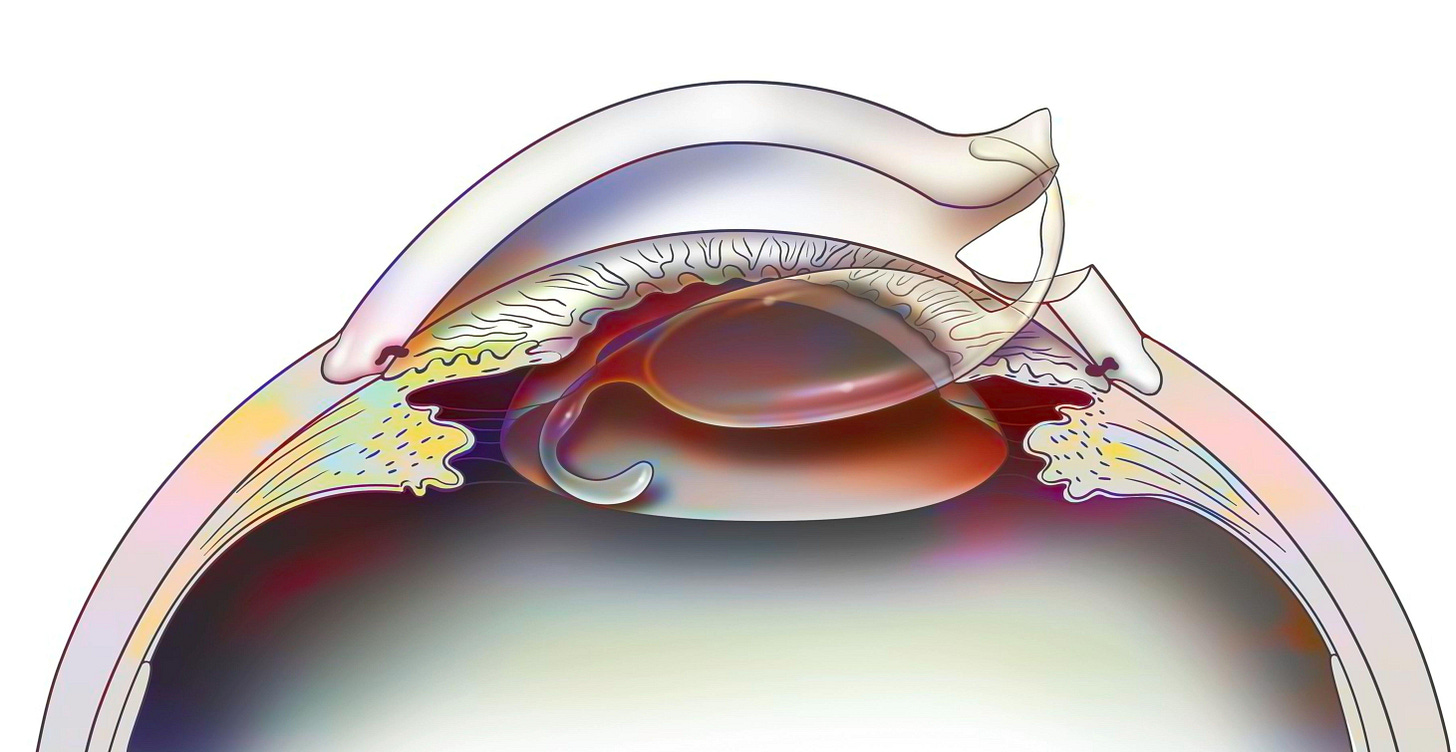
Although extractions had become more prevalent in the Islamic world, couching remained the primary cataract treatment in Europe. But in 1747, Jacques Daviel, a French military surgeon, introduced a completely new approach — extracapsular cataract extraction (ECCE). In it, Daviel made a precise incision near the limbus of the eye, the border between the cornea and the white part of the eye. Then he opened up the anterior capsule (front covering) of the lens, and carefully extracted the opaque material while preserving the posterior capsule (back covering) of the lens.
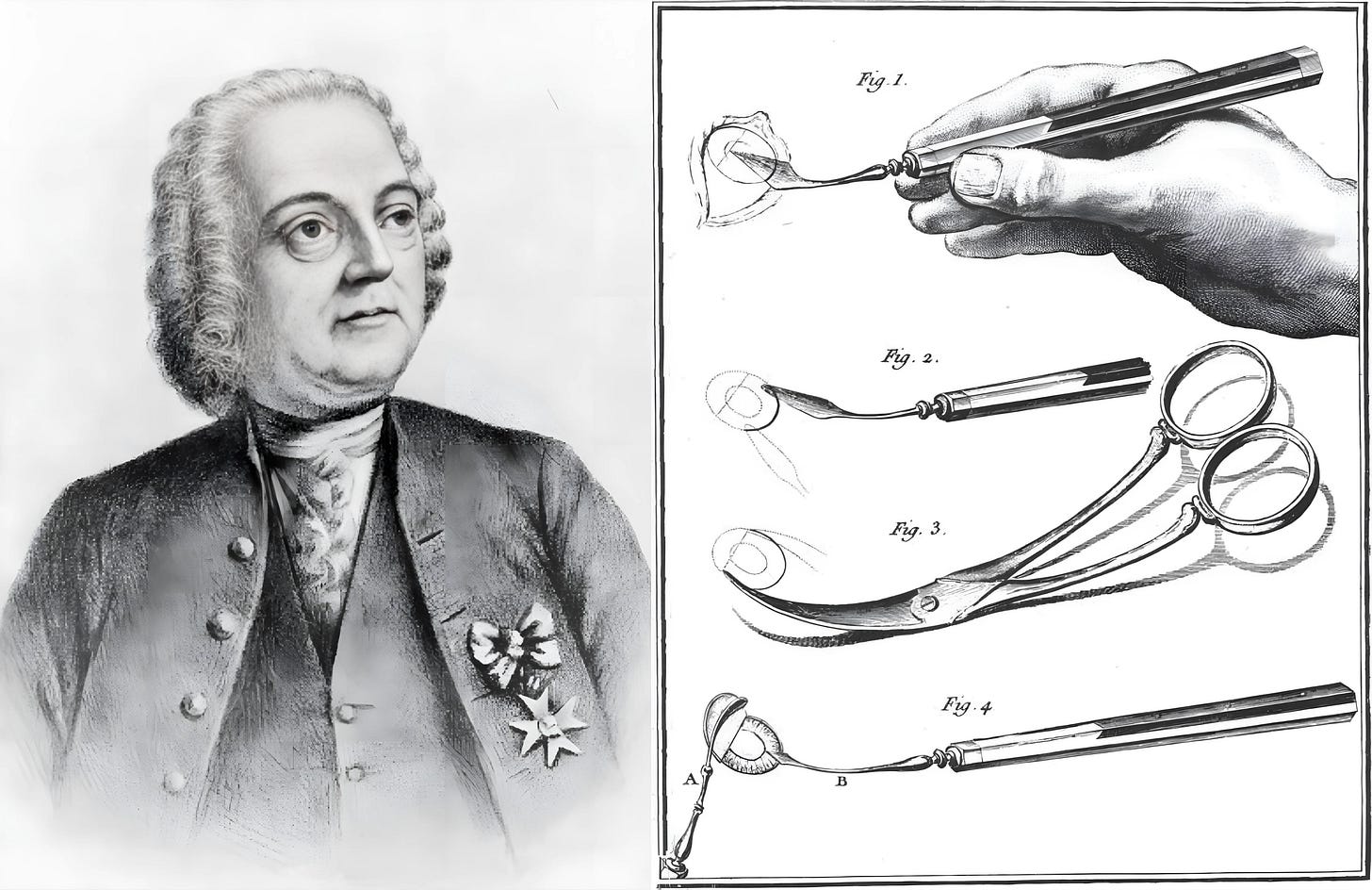
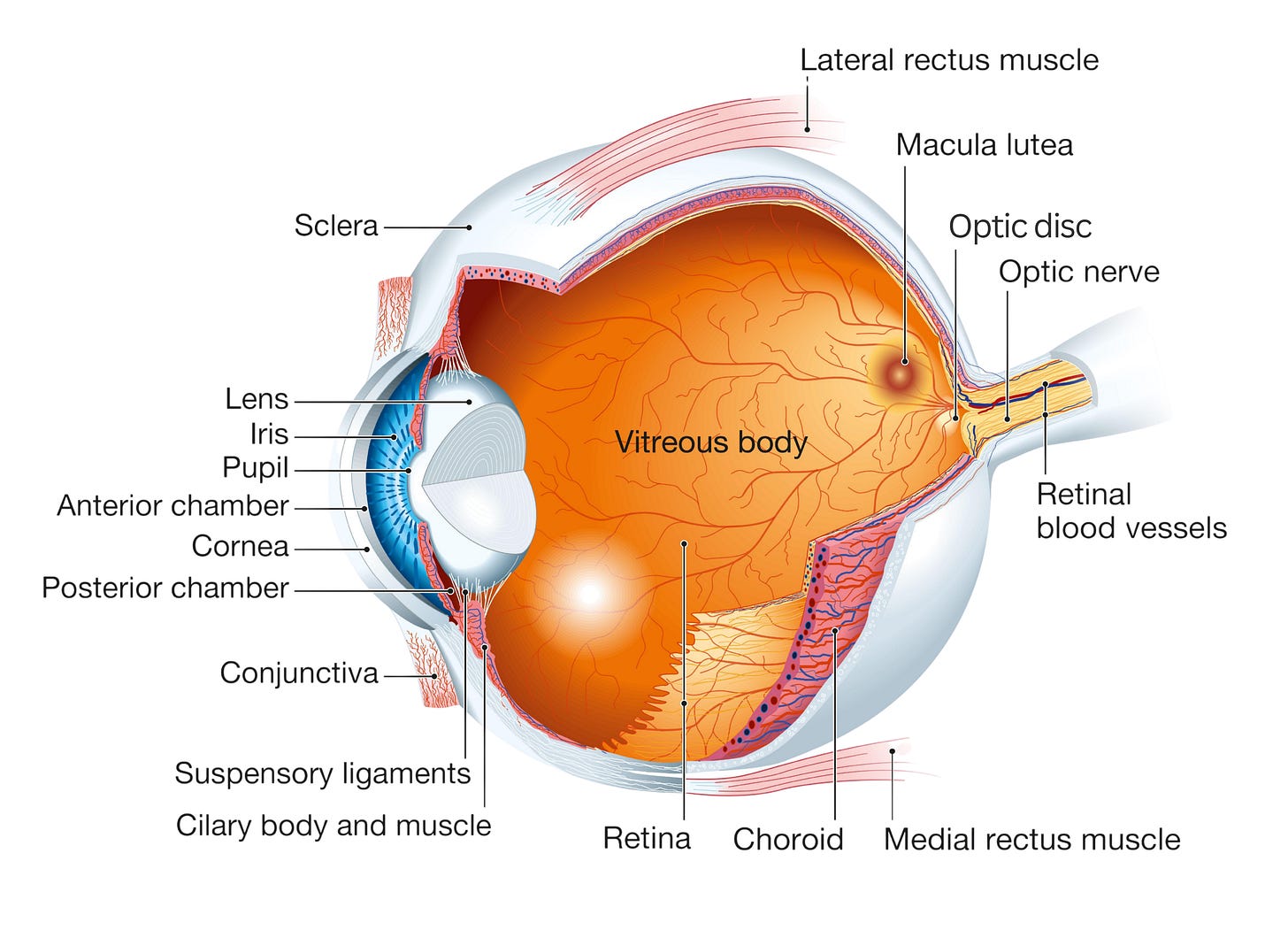
To appreciate the significance of this approach, it is important to understand the anatomy involved. During cataract surgery, the cloudy lens rests in front of the vitreous body, a clear, jelly-like substance filling most of the eye. The retina, responsible for vision, attaches to the back of the eye, behind the vitreous. If the posterior capsule is broken, the vitreous can move forward and disturb the delicate retina, increasing the risk of vision loss. By keeping the posterior capsule intact, the surgeon preserved both the vitreous and the posterior chamber, the space just behind the lens, thereby maintaining the eye’s natural structure and function. Daviel’s preservation of the posterior capsule is still followed in cataract operations today.
As with many medical revolutions, however, progress was not linear. Just three years later, in 1750, a British surgeon named Samuel Sharp proposed yet another method, called intracapsular cataract extraction (ICCE). In this technique, the entire lens, including the lens capsule (a thin, elastic membrane that holds the lens in place), is removed from the eye. Sharp used a corneal knife to make an incision and then compressed the eyeball with his thumb to expel the intact lens, without opening the capsule.
Sharp’s method was mechanically simpler than Daviel’s. By removing the intact lens, the surgeon avoided disturbing the eye’s delicate internal structure. He detailed this procedure before the Royal Society on November 22, 1753, describing nineteen operations in patients ranging from 47 to 70 years old, of which thirteen resulted in good visual recovery. The five patients who had undergone surgery in both their eyes could even “read and write with proper spectacles.”
Interestingly, Sharp admitted that his technique, while an improvement upon Daviel’s, had its shortcomings. In his account to the Royal Society, Sharp conceded that, of all 19 operations, “There was not one [patient] that escaped inflammation.” He was also forthright about his mistakes, such as when he accidentally nicked a patient’s iris, “which bled profusely” (though he was careful to note that Daviel hadn’t observed any poor outcomes from such an error). He even ends with the admission: “I presume a greater number of operations will prove this account very deficient, but I have here communicated all that I have done … It is to be hoped that, when it shall be more generally practiced, ingenious men will render it still more perfect.”
Perhaps the largest “deficiency” of both Daviel and Sharp’s techniques was that patients who underwent them were left “aphakic,” with their natural lens gone and their vision blurred. With the help of thick convex spectacles, their sight was often clearer than it would have been with couching, but these glasses were bulky and still left the wearer with distorted sight and limited peripheral vision.
The lens, after all, plays a central role in vision by bending and focusing light. If it’s removed without being replaced, the eye loses its focusing power (communicated in “diopters,” a measurement of how strongly a lens refracts light). Without lenses, then, a person’s vision might drop by as much as 20 diopters, leaving them horrendously farsighted.
So while Daviel and Sharp recognized the importance of actually removing cataracts, they had not yet hit upon a means of replacement.
Intraocular Lens Implantation
This next pivotal advancement emerged during WWII, when Harold Ridley, a British ophthalmologist serving as a consultant to the Royal Air Force, encountered a series of fighter pilots who had sustained ocular injuries from shattered cockpit canopies. Often, these injuries left fragments of the canopy material embedded in their eyes.
These fragments were made of polymethyl methacrylate, a lightweight, transparent plastic that, much to Ridley’s surprise, did not provoke the eye irritation that would normally be expected from foreign material. Intrigued, Ridley wondered whether a synthetic material similar to PMMA could be used to fashion an artificial lens to restore optical function after cataract extraction.4
With the help of his friend John Pike, a scientist at the optical devices company Rayner and Keeler, Ridley devised a design for the first intraocular lens (IOL). Meanwhile, Pike enlisted Dr. John Holt of the chemical company Imperial Chemical Industries to produce a medical-grade version of PMMA free from impurities. They used the resulting material, eventually called Perspex Clinical Quality (CQ), to prototype the world’s first IOL.5
The next step was to test this lens in a patient. Ridley sought one with a cataract in a single eye but with functional vision in the other so that, should the IOL implantation fail, their quality of life would not be severely impacted. It took almost a year before Ridley found Elizabeth Attfield, a 45-year-old hospital nurse with a cataract in just her left eye.
For the operation, Rayner and Keeler manufactured a simple disc-shaped lens with a peripheral groove from the specially prepared Perspex CQ, providing it to Ridley at a cost of just eighteen shillings (about £38 today). On November 29, 1949, Ridley performed an extracapsular cataract extraction on Attfield. He then waited three months to ensure the eye had tolerated the extraction before implanting the first IOL, which he carefully placed behind the iris onto the front of the posterior capsule.
The outcome of this first surgery was mixed. Attfield's central visual acuity improved to 20/60,6 but her vision shifted -14 diopters. This shift happened because the implanted lens design was a bit too thick. As such, it did not account for the difference in refractive index — how much a material bends light — between the artificial lens and her natural lens, causing a refractive error.
An implantation on a second patient in August 1950 resulted in a change of –15 diopters. After modifying the curvature of the artificial lens surface, which determines its focusing power, Ridley’s third IOL in November 1950 achieved a much more acceptable result of +4.5 diopters, closely matching the patient’s other eye.
In Ridley’s early surgeries, no suture was used to hold the eye structures in place, and part of the iris, the colored portion of the eye that controls pupil size, slipped out through the surgical incision, a complication known as iris prolapse. Ridley corrected this in later procedures by adding a corner stitch to keep the chamber stable. In another case, the lens shifted downward inside the eye because it was too heavy and lacked proper support. This drove Ridley to create a lighter lens.
Ridley waited for 18 months after his first IOL implantation before presenting his findings, hoping to showcase two-year follow-up results to prove the procedure’s safety and efficacy. However, an unexpected coincidence forced his hand. One of his patients, consulting the telephone directory for his post-operative visit, found a “Dr. Ridley” listed, made an appointment, and arrived at the address, only to discover he was at the office of Dr. Frederick Ridley, a different ophthalmologist. After this encounter, the news quickly spread.
In July 1951, Ridley presented his work on IOLs at the Oxford Ophthalmological Congress, held at the School of Geology (with rooms at Frewin Hall for “lady ophthalmologists”). Though Ridley presented his results sooner than he would have liked, he had by that time performed IOL implantation in eight patients. He brought two of his most successful patients along as evidence of his triumph, one with vision restored to 20/20 (considered the normal standard) and the other achieving 20/15, even better.
Despite a smattering of polite applause and a few questions, Ridley’s presentation quickly turned sour. Notably, Sir Stewart Duke-Elder, the doyen of British ophthalmology, refused even to examine Ridley’s patients, citing the absence of preclinical animal trials and the experimental nature of the procedure. There may have also been a layer of professional jealousy, as Duke-Elder had also been working at the Moorfields Eye Hospital in London and even attended to some of the same patients before passing them off to Ridley. One of Ridley’s biographers, David Apple, observed that he must have felt foolish:
“There he was, the greatest and most influential of all British ophthalmologists, the ‘ophthalmic Aladdin’ as he was sometimes called. He had not seen and made the connection as to what may have been right before his eyes — the idea to invent the IOL.”
Ridley published the results of further studies in 1952, but was met with a similarly cold reception. The rejection was particularly sharp in Britain, undoubtedly owing to Sir Duke-Elder’s influence, where ophthalmologists voiced critiques, such as "[Ridley’s] first report was a layman's magazine" and "This operation should never be done." Only a few surgeons, such as Peter Choyce and Edward Epstein, offered support. Overseas, however, the reception was warmer. The Soviet surgeon Svyatoslav Fyodorov, after learning of the operation, traveled to England to meet Ridley and observe the procedure, becoming a lifelong supporter of his work.
Resistance started to wane in the 1970s, by which point thousands of modified lens designs had been successfully implanted across Europe and in the United States. And by the 1974 International Congress of Ophthalmology in Paris, following presentations on advancements in complementary techniques, IOLs appeared to be gaining traction. Dr. Robert Drews of St. Louis, Missouri, a prominent American cataract IOL surgeon who had attended, reflected that: “Five thousand years, 250 years, 40 years ago; what will the future hold? I think the time is coming when information processing will advance to the state that we can begin to make inroads in providing pseudo-visual input for the blind. But no matter what brilliant achievements are made in the future, Ridley's place in history remains secure.”
Precision Era
The advancement in cataract surgery that was truly responsible for “establishing a bright, new world for IOLs” had actually arrived in the 1960s. It didn’t come from war or a surgical textbook but a dentist’s chair. Charles D. Kelman, an American ophthalmologist, was undergoing a dental cleaning involving ultrasonic instruments designed to remove plaque with minimal mechanical force. Incidentally, Kelman was only at the dentist because he was so despondent about failed attempts7 to improve cataract surgery that he had let himself fall to pieces: “I had, meanwhile, allowed my hair to grow down to my shoulders, and my teeth badly needed a cleaning.”
The hum of this scaler sparked an idea that would propel cataract surgery into its modern form: What if, he thought, a cataract could be emulsified — broken into tiny particles to be more easily removed from the eye? This would minimize trauma to the eye, speed up recovery, and reduce postoperative complications.
His first device, made by the ultrasonic scaling device company Cavitron Corporation, was rudimentary but serviceable. It consisted of a large boxy unit that housed the machine components, and a wand-like instrument that the surgeon would grip like a pencil. At the tip of this handpiece was an ultrasonic probe much like the one the dentist had been using.
By 1967, after conducting animal trials, Dr. Charles Kelman was ready to test his “phacoemulsification machine” on a blind patient, who elected to undergo the surgery voluntarily. The operation took more than four hours to complete, with the ultrasound step alone lasting sixty-one minutes. The earliest “phaco handpiece” was much heavier than today’s versions, making the operation tiring and difficult to control. Unfortunately, the patient’s eye became infected after surgery and had to be removed. In fact, Kelman’s earliest attempts were fraught with such complications, and he spent two years seeking a way to prevent corneal collapse.
But Kelman persisted. He refined his instrumentation, collaborated with engineers to create safer, more efficient phaco tips,8 and meticulously documented his clinical outcomes. By 1970, he felt confident enough to train other surgeons in his improved technique, organizing courses in New York. His new methods drastically reduced the size of the surgical wound from 10 to 12 millimeters to under 3 millimeters. This meant fewer stitches, reducing induced astigmatism and enabling earlier visual rehabilitation.
Throughout the 1980s and 90s, advances in ultrasound tip design, fluidics control, and foldable intraocular lenses9 made phacoemulsification even safer and less invasive, allowing much smaller incisions that no longer required sutures. The 2000s brought refinements in torsional and transverse ultrasound (which emulsify the lens more efficiently with less heat),10 and the integration of real-time computer control for stability and precision.
By the 21st century, the field had fully entered its precision era, marked by the introduction of laser surgery. In 2008, Hungarian ophthalmologist Zoltan Nagy performed the world’s first Femtosecond-Laser-Assisted Cataract Surgery (FLACS) procedure using a femtosecond laser, a specialized surgical instrument that emits ultrashort pulses of light.11 While used in operations, more traditional means of phacoemulsification remain standard practice.12
Today, cataract surgery begins with the patient receiving topical anesthetic eye drops or a small amount of local anesthetic around the eye. Next, the surgeon creates a tiny incision in the cornea, only about 2-3 millimeters wide. It is through this incision that phacoemulsification takes place as surgeons insert an ultrasonic probe that vibrates at an astonishing rate of 40,000 times per second. The cataract is broken into tiny fragments and washed out of the eye with irrigation and suction, leaving behind the clear capsular bag that originally held the natural lens.
With the structure of the bag preserved thanks to surgical techniques from the likes of Daviel, the next step is the placement of an IOL. Modern IOLs are foldable, allowing the surgeon to insert them through the same small incision used in the phacoemulsification stage using a special injector. Once inside the eye, the lens unfolds neatly into the capsular bag. The whole process takes about 20 minutes, and most patients experience improved vision within hours to days, with full healing typically complete within 4-6 weeks.
There is variation, of course, with some surgeries using slightly different phacoemulsification techniques such as FLACS or different IOL designs. For example, a type of IOL known as “light-adjustable lenses” (LALs) can be fine-tuned after surgery using ultraviolet light. They are made from a special photosensitive silicone polymer containing light-reactive macromers (essentially small chains of molecules with “loose ends” capable of linking up with other chains when triggered). After surgery, when the eye has stabilized, the patient sits at a slit-lamp–mounted device that shines targeted UV light (of around 365 nm) onto the IOL. Wherever the UV strikes, the macromers polymerize, altering the lens curvature and refractive index. Once the surgeon is confident they have addressed any residual visual distortion, they apply a final UV exposure that polymerizes all remaining macromers, permanently stabilizing the lens.
Regardless of differences or personalization of techniques, such as that granted by LALs, cataract surgery is no longer a question of whether vision can be restored at all, but how well.
A Vision of Clarity
Of course, this is not the case for everyone. Despite being one of the most successful medical interventions, cataract surgery remains inaccessible to vast segments of the world’s population due to inequities in infrastructure, personnel, and financing.
Cataract Surgical Rate (CSR) represents the number of cataract operations performed per million people annually and serves to measure a health system’s capacity to address avoidable blindness. In high-income countries such as those in North America and Europe, CSR typically ranges from 5,000 to 10,000 per million annually, while in sub-Saharan Africa, it often falls below 500 per million. Countries like China and India report CSRs exceeding 10,000 per million, due to large-scale national programs.
Countries with low CSRs face barriers such as limited training infrastructure, inadequate financial support, and a steady outflow of skilled professionals, drawn abroad by better salaries, education, and working conditions. A 2015 study showed that while some high-income countries have 76 ophthalmologists per million people, many low and middle-income countries experience ratios as low as 3.7 per million. Even if the latter have cataract surgery programs, waiting times can stretch into months, often leading to irreversible visual disability by the time surgery can be performed.13
While the cost of cataract surgery itself is relatively low by international standards, estimated between $71 for outreach cases in Nigeria to $299 in other parts of Africa, this figure often excludes several hidden costs that significantly impact affordability. For example, in Madagascar, where over 80 percent of the population lives on less than $1.90 a day, a single bus ticket between cities can cost nearly $25. Surgical patients frequently need to travel even greater distances and, often, multiple times for follow-up. Thus, travel expenses alone may represent more than a month’s income for many households.14
There is also an imbalance between the sexes when it comes to accessing surgery. Even though women suffer from cataracts at a higher rate than men, women often lack the financial autonomy needed to pursue care, or the treatment of male family members takes precedence. Women can also face difficulties traveling to clinics in the absence of a companion. And additional barriers, such as stigma and fear of surgery, can also disproportionately hinder women’s access to treatment.
Disparities in access not only take a toll on public health but also on the economy. The global productivity loss due to vision impairment is estimated at $411 billion annually. Cataracts account for a large fraction of this burden, especially in individuals between 50 and 75 years of age who are either still working themselves or role as a caregiver allows other family members to work. Thus, cataract surgery can not only change a single patient’s life but also the economic trajectory of their entire household.15
Fortunately, these challenges are surmountable. In my more than five years of experience as a consultant ophthalmologist at Aravind Eye Hospital in Madurai, Tamil Nadu, I’ve seen various successful approaches to expanding access to cataract surgery and reducing the disparity of care in low-resource settings.

Aravind Eye Hospital was founded in 1976. Today, it is one of the largest eye care providers in the world, with a network of 14 hospitals across South India. The surrounding state is historically one of India’s poorest regions.
At the heart of Aravind is a hybrid, self-funding business model. Roughly half its patients pay market rates for care, which subsidizes free or at-cost services for the other half. Reducing costs beyond the surgery itself has been just as important.
For example, Aravind identified that the cost of IOLs (usually around $100 per lens) presented a significant barrier in low-resource settings. To overcome this, Aravind began to manufacture its own. The result was Aurolab, a manufacturer established in 1992, which reduced the price of each lens to below U.S. $10. Clinically, these lenses match the safety and optical quality of imported versions, the main difference being that they are rigid PMMA lenses rather than the foldable acrylic ones used in high-income countries. Today, Aurolab lenses are exported to more than 140 countries.
Even with lowered costs, hospitals and clinics face challenges connecting with prospective patients. In the late 1970s, less than 15 percent of people in Aravind’s service area who needed eye care were actively seeking it. The hospital network targeted the remaining 85 percent by increasing outreach.16 By working with social organizations, NGOs, volunteers, and schools, Aravind began to organize screening camps. Each year, these camps serve over half a million people, with more than one-third receiving interventions they would never otherwise have gotten.
Similar outreach efforts occur across many low-income countries. Multiple organizations, including NGOs like Sightsavers, Lions Clubs, and HelpAge India, play a large role in organizing eye screening camps and mobilizing communities to connect clinics with patients. In countries such as Bangladesh, Gambia, and Tanzania, outreach camps, community mobilization, and public-private partnerships work with NGOs to provide awareness campaigns and follow-up care.
Finally, more trained personnel are needed. Aravind uses a two-pronged approach to addressing the lack of ophthalmologists: First, it enhances the efficiency of the existing staff. The hospital has an innovative “assembly line” operating theatre that allows a single surgeon to alternate between two fully prepared tables, each supported by dedicated instrument sets and nursing teams. This approach enables six to eight cataract operations per hour compared to an industry norm of one, while delivering clinical outcomes that even surpass those achieved in the UK’s National Health Service.
Second, it expands its training efforts. At Aravind, nurses and technicians, drawn largely from local communities and often women with no prior medical background, are trained to function as certified ophthalmic assistants. Representing about 60 percent of the workforce, these cross-trained staff handle multiple roles, from preliminary examinations to surgical preparation.17
Regardless of what individual agencies, clinics, and hospitals do, more governments should play a larger role in making eye health more widely accessible. India’s central government runs the National Program for Control of Blindness and Visual Impairment (NPCBVI), which includes major subsidies for cataract surgeries and the aforementioned screening and surgical campaigns. Currently, programs like “Netra Jyoti Abhiyan” aim to clear the national cataract backlog by subsidizing millions of surgeries annually. The Vision 2020 initiative significantly increased cataract surgery rates in Africa, with regions like Kenya’s Kwale and Tanzania’s Kilimanjaro nearly doubling their surgical rates through community screening and the expansion of trained staff. And in Latin America, Brazil’s federal mass campaign efforts have greatly reduced cataract blindness.
Ultimately, efforts such as these acknowledge the fact that a successful cataract program is a clear public good, a clinical intervention with enormous implications for the economy and global health. It is not measured only in restored sight but in restored agency: the grandmother who reads again, the laborer who returns to work, the child who need not drop out of school to guide a blind parent.
Technology, including biomedicine, achieves its greatest impact when delivered equitably. This does not mean everyone in the world must have access to exactly the same methods of care, but it should mean we strive to extend the “triumphs of science” to as many people as possible. The ability to restore vision is a measure of clinical brilliance. Making it universal is the measure of our humanity.
Dr. Sangeetha Aravinda is an ophthalmologist and consultant at Aravind Eye Hospital, currently residing in Thanjavur, Tamil Nadu, India. She holds distinctions in microbiology and community medicine and earned honors in pharmacology during her undergraduate medical training. She is a passionate reader and teacher of ophthalmology, actively engaged in mentoring juniors and mid-level ophthalmic personnel. She is the author of two books, Mastering Glaucoma and Mastering Uvea, published on Amazon Kindle, and continues to foster a love of clinical learning in her community.
Thanks to Xander Balwit for extensive guidance and support. Thanks also to my husband, Dr. Aravinda, an academic and public health physician, for his encouragement and thoughtful feedback throughout the writing of this article.
Lead image by Ella Watkins-Dulaney.
Cite: Aravinda, Sangeetha. “The World’s Most Common Surgery.” Asimov Press (2025). https://doi.org/10.62211/72kj-84pk
The eye is an immune-privileged site, a concept first described by Sir Peter Medawar in the 1940s, when he observed that foreign tissue grafts placed in the anterior chamber of the eye were not rejected. An example of this is corneal transplantation, which achieves success rates of up to 90 percent even without tissue matching. A major breakthrough in ocular gene therapy came in 2017 when the FDA approved voretigene neparvovec (Luxturna), the first gene therapy for inherited retinal diseases caused by RPE65 mutations.
Trachoma is a contagious bacterial infection often passed from child to child and from child to mother, leading to blindness when eyelids turn inwards and scrape the cornea. It affects nearly 2 million people worldwide and is especially endemic in developing countries. While the blindness from Chlamydia trachomatis is irreversible, it can be avoided with a simple surgery costing under $100, making it a popular target of cost-effective charitable donations.
Despite many years of research into pharmacological methods such as antioxidants, aldose reductase inhibitors, and sulfhydryl agents, no treatment has successfully stopped the progression of cataracts other than surgical intervention.
Before this, artificial eyes had been used mostly for appearance. The earliest known prosthetic eye, dated to around 2,900 to 2,800 BC, was found in a burial site in Iran. It was made from bitumen paste and covered with a thin layer of gold. Later, around 500 BC, Egyptian and Roman priests made painted clay eyes to tie over the eyes of the deceased, believing that this allowed them to see the offerings left by their families and maintain relationships with the living.
Remarkably, the men chose not to patent the lens, prioritizing patient benefit over commercial gain.
These numbers come from the Snellen Chart (commonly found in optometrist offices), named after the Dutch ophthalmologist Herman Snellen. The higher the denominator, the weaker your vision: 20/60 means that at 20 feet, you see what a normal eye would see at 60 feet.
According to his obituary from the American Academy of Opthamology, Kelman “had tried some 40 prototypes in his quest to dissolve the lens during cataract surgery,” including frozen probes and meat-grinder type devices.
Upgrades included lighter instruments, titanium tips to prevent flaking, and devices capable of irrigation and aspiration.
The first foldable IOL to receive FDA approval was the silicone lens by Allergan in 1990. Made from solid silicone polymers, it demonstrated biocompatibility that was superior to Ridley’s earlier IOLs.
This had already improved a great deal from Kelman’s early prototypes, which essentially boiled the eyes of the cats he tested his early devices on.
Unlike traditional phacoemulsification, where all steps of the procedure were performed manually and were heavily dependent on the surgeon’s skill, FLACS employed computer-guided lasers to perform these tasks with far greater reproducibility. The laser created micron-precise incisions in the cornea, opened the lens capsule in a perfectly circular pattern, and pre-softened the cataract for easier emulsification.
As with previous innovations, critics have argued that the benefits of FLACS, such as slightly improved centration of the lens or marginally reduced ultrasound energy, did not justify its increased cost (about U.S. $600 more than phacoemulsification) and infrastructure demands (as operating a femtosecond laser required a dedicated room and specially trained technicians).
Patients who wait more than six months for cataract surgery often experience greater vision loss, poorer quality of life, and a higher risk of falls compared to those treated within six weeks.
Beyond transportation, patients must also pay for food, accommodation, and postoperative visits.
When an individual with cataracts has successful surgery, it is estimated to avert 0.85 DALYs, which means the person regains about 0.85 years, or roughly ten months, of healthy life that would have been lost due to vision problems. DALY stands for “Disability-Adjusted Life Year.” It is a measure used in public health to capture the overall burden of disease and takes into account both the years lost due to early death and the years lived with disability or poor health because of a disease.
Beyond in-person outreach, Aravind also makes use of telemedicine-focused vision centers. These centers are embedded in local communities but connected digitally to base hospitals, enabling remote diagnosis by specialists while reducing the need for patient travel. They are contingent on internet access, and their success is constrained by patients that are then able to seek treatment, but they still provide a valuable first line of diagnosis and education.
Such community training has happened elsewhere as well. For example, in Sub-Saharan Africa, the Kilimanjaro Centre for Community Ophthalmology (KCCO) offers extensive training for non-physician cataract surgeons, as well as programs for ophthalmic nurses and allied health personnel.



I've had lens replacements with cataract surgery. I must have missed his telling me that during the preop briefing. I was astounded by the results. My vision went from 20/400 to 20/20 almost overnight. My vision is normal after living my entire life wearing thick glasses. "Life changing" is a very overused term these days, but for me it literally was. Hail science!
Incredible article, and inspiring. Thanks so much for writing it!