An Antivenom Cocktail, Made by a Llama
A new broad-coverage antivenom, made by mixing eight different nanobodies, protects mice against snakebites from 17 of 18 deadly species in Africa.
Growing up, my family’s llamas never did anything useful. There was no need for Andean pack animals on their small acreage farm in Oregon wine country, so the creatures simply idled on my grandparents’ property, spitting at us and occasionally getting stuck in the pond. Looking back, it is hard to imagine that these beasts bear any resemblance to the llamas at the center of a recent study on making better antivenom — yet they do.
Today, researchers reported in Nature how a llama and an alpaca were injected with venoms from 18 of the most deadly snakes on the African continent to make a broad-spectrum antivenom, the product of a vast amount of experimental work, spanning years of effort.1 By isolating antibodies from these animals, expressing more than 3,000 recombinantly in engineered E. coli cells, and combining eight, the resultant antivenom prevented venom-induced death in mice injected with 17 of Africa’s most lethal elapid snake venoms. And while this antivenom has yet to move into phase I trials, it offers greater protection to mice than Inoserp PAN-Africa, a commercial antivenom approved by the WHO that was specifically developed for snakes found in sub-Saharan Africa.2
Although a recent computational approach for a designed antivenom by the Baker group at the Institute for Protein Design has garnered significant attention in the past year, this paper underscores that there is still much value that can be extracted from traditional antibody development techniques. By injecting animals with the full slate of diverse toxins present within venom and then screening thousands of antibodies to identify which ones bind to and neutralize a wider range of damaging toxin subfamilies — not only the three-finger toxins (3FTxs) at the center of Baker’s work but also phospholipase A₂ (PLA₂) and Kunitz-type serine protease inhibitors (KUNs) — it was possible to create a potent antivenom that protects against multiple toxin types.
The paper thus offers a promising solution to what has been dubbed “the antivenom crisis;” a truly broad, manufacturable antivenom cocktail that outperforms the best remedy currently on the market (at least in mice).
The Problem
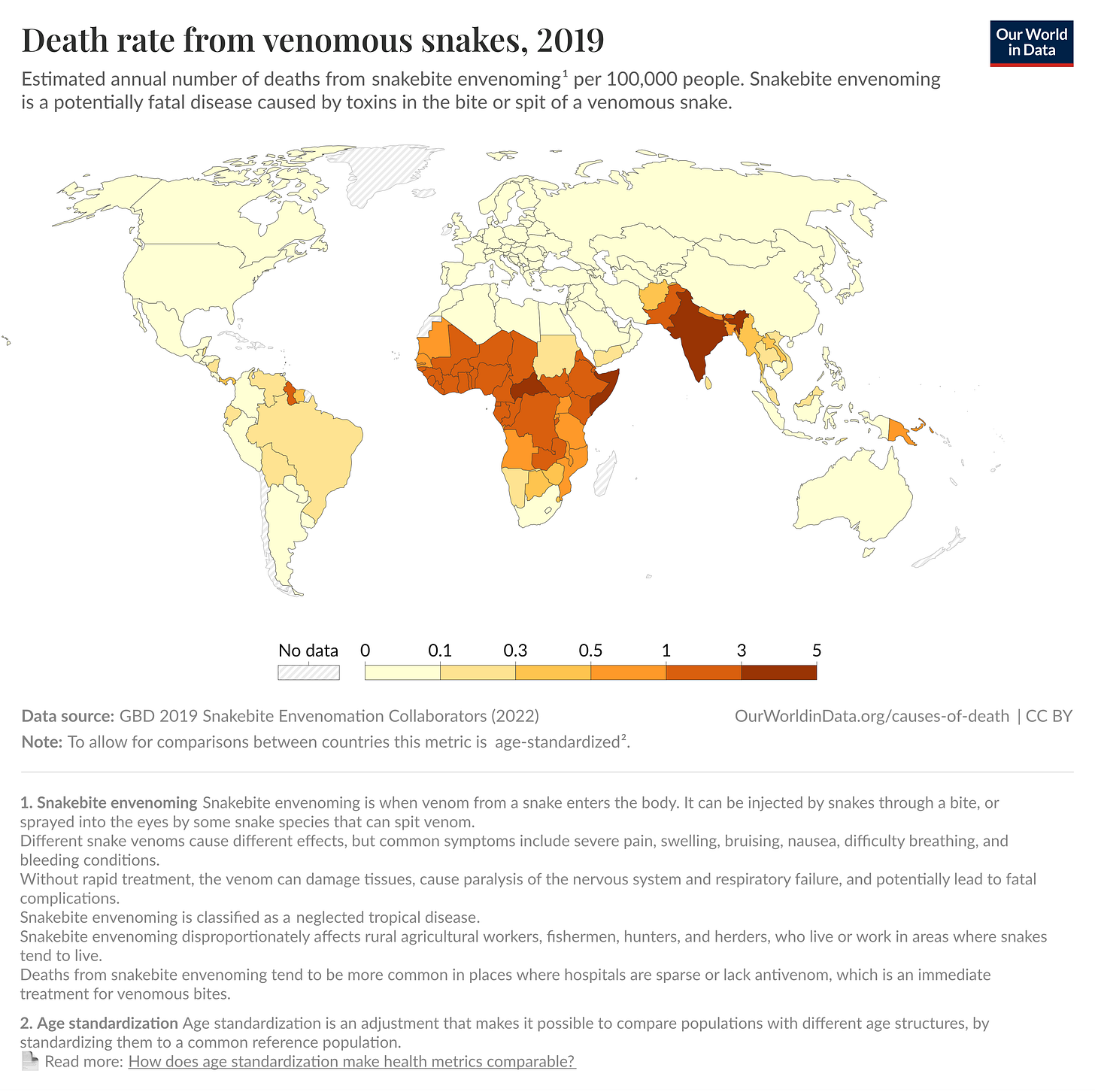
If ever there were a truly menacing snake family, it would be the elapids, characterized not only by a set of permanently erect frontal fangs but also a threat display involving rearing up and fanning out a neck flap. While elapids are found worldwide in tropical and subtropical regions, they pose an enormous problem in sub-Saharan Africa, where their bites cause 7,000 deaths and 10,000 amputations each year (though these are likely underestimates, because many deaths go unreported).
When biting, these snakes inject their venom through hollow or grooved fangs that work like hypodermic needles. Once inside the body, the venom spreads toxins via the lymphatic system and bloodstream.3 As they circulate, the various foreign proteins present in the venom target specific systems: neurotoxins attack the nervous system, causing paralysis and respiratory failure, hemotoxins destroy blood cells and damage blood vessels, leading to internal bleeding and tissue death, and cytotoxins break down cells at the bite site, causing severe local tissue damage and necrosis. While cowboy Westerns often depict the application of tobacco poultices or vigorous sucking and spitting to draw out the poison, antivenom is the only way to halt this cascade of cellular damage.
Antivenom is typically produced by immunizing horses or sheep with small amounts of snake venom and then harvesting and purifying their antibody-rich serum. When administered intravenously, this serum circulates rapidly throughout the bloodstream, while the antibodies within bind to venom molecules through highly specific protein-protein interactions, forming immune complexes that neutralize the toxins before they can rampage unchecked throughout the body.
The effectiveness of an antivenom depends critically on timing.4 It works best when administered within hours of the bite, before venom molecules have bound to their targets and caused irreversible damage. The problem, however, is that getting to a hospital quickly is often impossible. In a piece on the antivenom crisis from Works in Progress, author Mathias Kirk Bonde contrasts a suburban Australian seeking attention at a nearby clinic with what transpires in poor, rural regions in India:
For over 34 percent of Indian snakebite victims, it takes more than six hours to receive treatment. If the clinic lacks a cold chain — a coordinated system of temperature-controlled environments — it is limited to using antivenom that can survive room-temperature storage. Without equipment to diagnose the species of snake the patient was bitten by, doctors must use polyvalent antivenom … which is typically more expensive and less effective than species-specific monovalent antivenoms.
This excerpt underscores two major challenges facing antivenom: first, the problem of transport (both of the patient to the clinic and the antivenom to temperature-controlled storage), and second, the difficulties surrounding polyvalent antivenom (those effective against multiple species), which are not only more expensive but also less targeted. Because these polyvalent antivenoms contain antibodies against multiple snake venoms, patients must receive a much larger dose of foreign animal proteins than they would with monospecific antivenoms, significantly increasing the risk of adverse immune reactions.
Looming over all of this is an even more essential challenge: venom is a staggeringly complex biological substance. “Snake venom is not just one thing, but rather an umbrella term for a cocktail of proteins,” writes Abhishaike Mahajan in a blog post. “The therapeutic issue here is that the composition of the toxin can vary.” This variation can occur by snake families (elapids versus vipers), snake species (Black mamba versus Cape cobra), snakes of the same species (dictated by things like geographic location), and even within the same species over time (influenced by whether the animal is a juvenile or adult at the time of the bite). Each snake’s venom, in other words, includes a unique blend of harmful proteins.
Such complexity also helps explain why timing is not the only critical factor after someone has been bitten by a snake. Because of species-specificity, it also matters that they receive an antivenom that matches their snake’s particular venom composition. For example, while taipan antivenom (developed against the coastal taipan) effectively neutralizes the toxins of both coastal and inland taipans, it’s less effective against inland taipan venom. Therefore, snake-specific antivenoms (even between subspecies) are essential.

This bevy of challenges, however, has spurred researchers to explore a variety of ways to address the antivenom crisis.
Back in January, for example, David Baker and his team used a computational tool called RFDiffusion to create proteins that bind to, and neutralize, three-finger toxins, small toxic proteins whose name comes from their characteristic molecular structure resembling three fingers extending from a central core. Three-finger toxins bind to the receptors where motor neurons communicate with muscle fibers, thus blocking the electrical signals that allow muscles to move and causing a progressive paralysis that, if untreated, results in respiratory failure; the primary cause of death from elapid bites. When tested in mice, pre- or post-injection of the computer-designed binders completely protected animals from lethal neurotoxin doses, with full survival and no paralysis up to 24 hours after exposure.
This computational paper marked an important step toward solving some of the biggest challenges facing antivenoms. For one, the de novo proteins were smaller and simpler than antibodies, making them easier to manufacture using recombinant DNA technology (and avoiding the batch-to-batch variation that often limits the effectiveness of traditional antivenoms, made from serums.) Their small size also helps them penetrate tissue deeply to more quickly neutralize toxins. And finally, these proteins were explicitly designed to have a high thermal stability, meaning they can withstand higher temperatures without degrading or unfolding. This helps with antivenom transport and storage; they can be kept in warmer environments that may lack an adequate cold chain.
The limitation of Baker lab’s approach, however, is that, while it might help in effectively neutralizing three-finger toxins, it does little to fight the various other components at work within venom. It neglects a particularly sinister family of toxins known as the phospholipase A₂ (PLA₂) family, a group of enzymes that hydrolyze phospholipids in cell membranes, breaking apart the fat molecules that form the protective outer barrier of cells. In many elapid venoms (especially those found in spitting cobras and rinkhals), these PLA₂ toxins are major contributors to local tissue destruction, “dermonecrosis.” When venom doesn’t kill, but leaves the bitten individual maimed or suffering life-altering wounds, PLA₂ is often to blame.
For another study, published in Cell this past June, researchers extended their antivenom neutralization effort to include PLA₂. Here, a group led by Jacob Glanville and Peter Kwong identified two human antibodies recovered from a hyperimmune human donor who had self-immunized with hundreds of venoms that could neutralize toxins shared across many snake species. Structural studies revealed that these antibodies (dubbed “LNX-D09” and “SNX-B03”) block the same receptor-binding surfaces that the computationally-designed antivenom, made by Baker’s group, was targeting. When combined with varespladib, a small-molecule inhibitor that disables PLA₂, the resulting cocktail protected mice from lethal doses of venom from 19 medically important Elapid snakes (though not from the same geographic region).
Although this study provides yet another approach toward the creation of “universal antivenoms,” it, too, had limitations. For one, varespladib has a short half-life (about 5.5 hours in humans). So while it initially neutralized PLA₂, it didn’t remain in the bloodstream long enough to confer sufficient protection. For that, the researchers had to re-dose varespladib every eight hours,5 which is impractical for emergency treatment, especially in low-resource settings where continuous medical supervision isn’t feasible.
Desperately needed, then, is a broad antivenom able to work continuously across all of the deleterious toxic subfamilies and offer long-lasting protection in a medically realistic scenario.
Going Broader
The approach in the Nature study out today aims to provide just that. The researchers began with a process quite similar to traditional antivenom production, milking venom from 18 species of African elapids and injecting each of them into one llama and one alpaca. From here, they increased the dose of these venoms over 60 weeks, administering the animals with larger and larger doses of snake venoms over the span of more than a year. Once inside the camelids’ bodies, these venoms produced an immune response, and the animals began to make antibodies that could be withdrawn and interrogated for the particular traits these researchers sought.
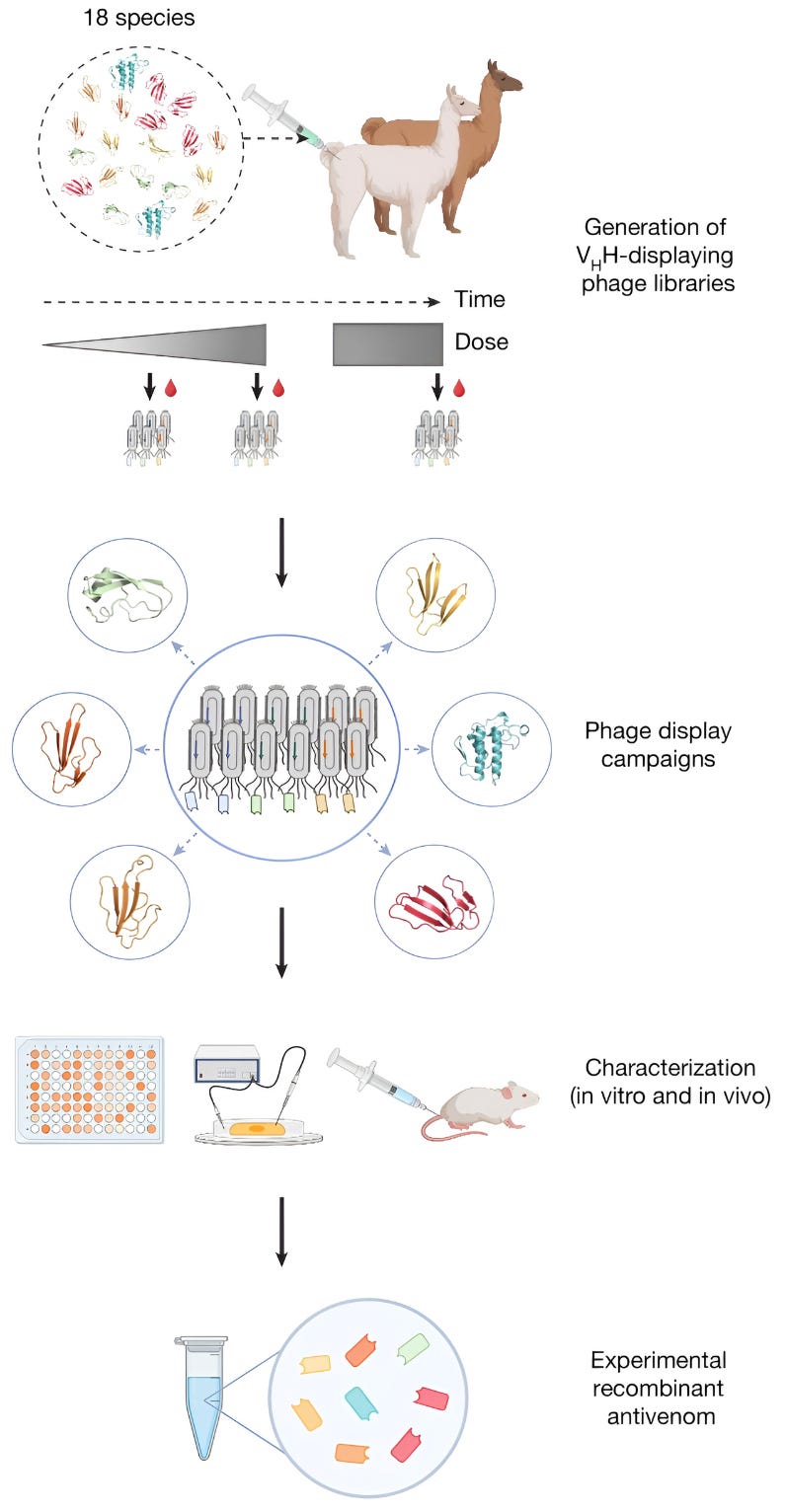
Next, the researchers used a technique called phage display to screen billions of antibody fragments and identify which ones bound best to various toxins within the venoms. Based on the results from the phage display, the researchers took 3,000 different antibodies and expressed each of them recombinantly in E. coli cells. The engineered microbes pumped out each of these antibodies, which were then isolated, one by one, and screened for binding against various toxins in the venoms. They found that 60 percent of the antibodies bound to their target toxin and more than 50 percent bound to multiple toxins from the same toxin subfamily. In other words, the llama and alpaca antibodies proved to be naturally promiscuous; a protein that neutralizes a three-finger toxin is likely to also neutralize other three-finger toxins in that same family.
After running some in vivo tests (involving injecting mice with antibodies that seem to have high cross-reactivity, and also that seem to bind their target toxins exceptionally tightly), they winnowed their antibody candidates down and combined just eight of them into an antivenom cocktail.
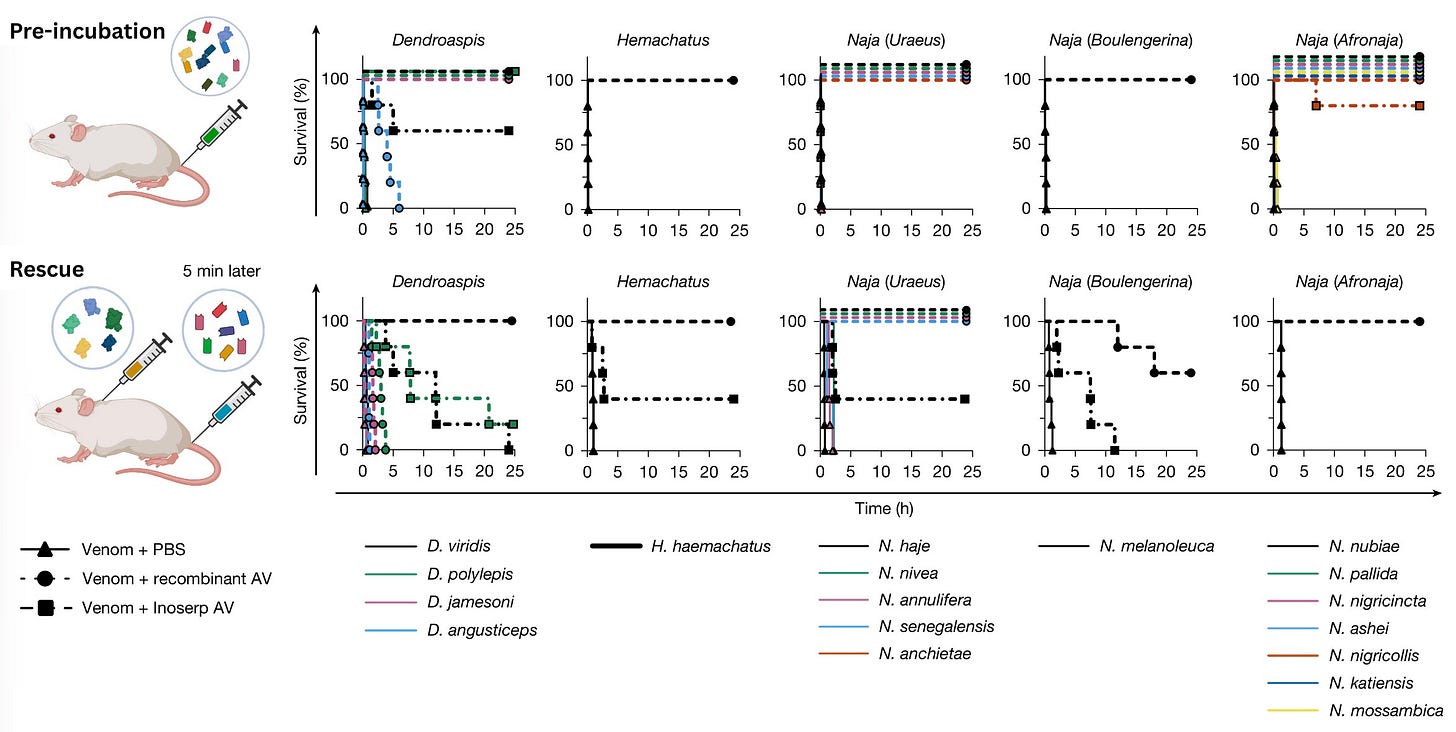
From here, the researchers conducted two sets of experiments. First, they did a pre-incubation experiment in which the antivenom cocktail was mixed with various snake venoms, and then the full mixture was injected into mice. Each animal was monitored for 24 hours to see whether they survived. This was not a “medically realistic” experiment, because the antivenom is given with the venoms. But the goal is merely to help establish baseline potency and answer the question of whether the antivenom will neutralize the toxins if it contacts them directly.
The researchers observed no signs of envenoming for 13 out of 18 of the snakes whose venom was injected during the pre-incubation work. For two of the snakes, pre-incubation with the antivenom still led to “minor signs of envenoming, including closed eyes and periods of lethargy interspersed with periods of excessive grooming,” but the animals survived. Two other species had similar late-onset symptoms, but likewise recovered. Overall, minor signs of envenoming were observed in mice injected with venom from 4 out of 18 snakes. The only snake that the cocktail failed to protect against was D. Angusticeps, the Eastern green mamba, a thin neon snake harrowingly nicknamed the “people-chaser.”6 However, even the mice injected with this cocktail survived twice as long as they would if injected with the venom alone (from three hours to six hours of survival).

The next experiments were the “rescue experiments,” so named because the antivenom is administered after exposure to the venom (more realistically what would occur after envenoming). Each mouse was injected with three-times the lethal dose of a given snake venom, and then treated with antivenom five minutes later. Once again, the mice were monitored for 24 hours.
The researchers benchmarked both experiments against the commercially available Inoserp PAN-AFRICA, with the manufacturer’s recommended dose similarly scaled up to contend with three times the median lethal dose of venom. The recombinant antivenom completely prevented lethality for 6 of the snake venoms in the rescue experiments, with no signs of envenoming. The Inoserp PAN-AFRICA antivenom, by contrast, “showed only partial neutralization of all the tested venoms and an extension of time of death for the venom from N. melanoleuca.” In other words, the recombinant antivenom “performed better than the commercial antivenom on all included venoms at the tested doses.
Toward Synthetic, Broad Antivenoms
There are several reasons to think that this new approach might help in addressing the antivenom crisis.
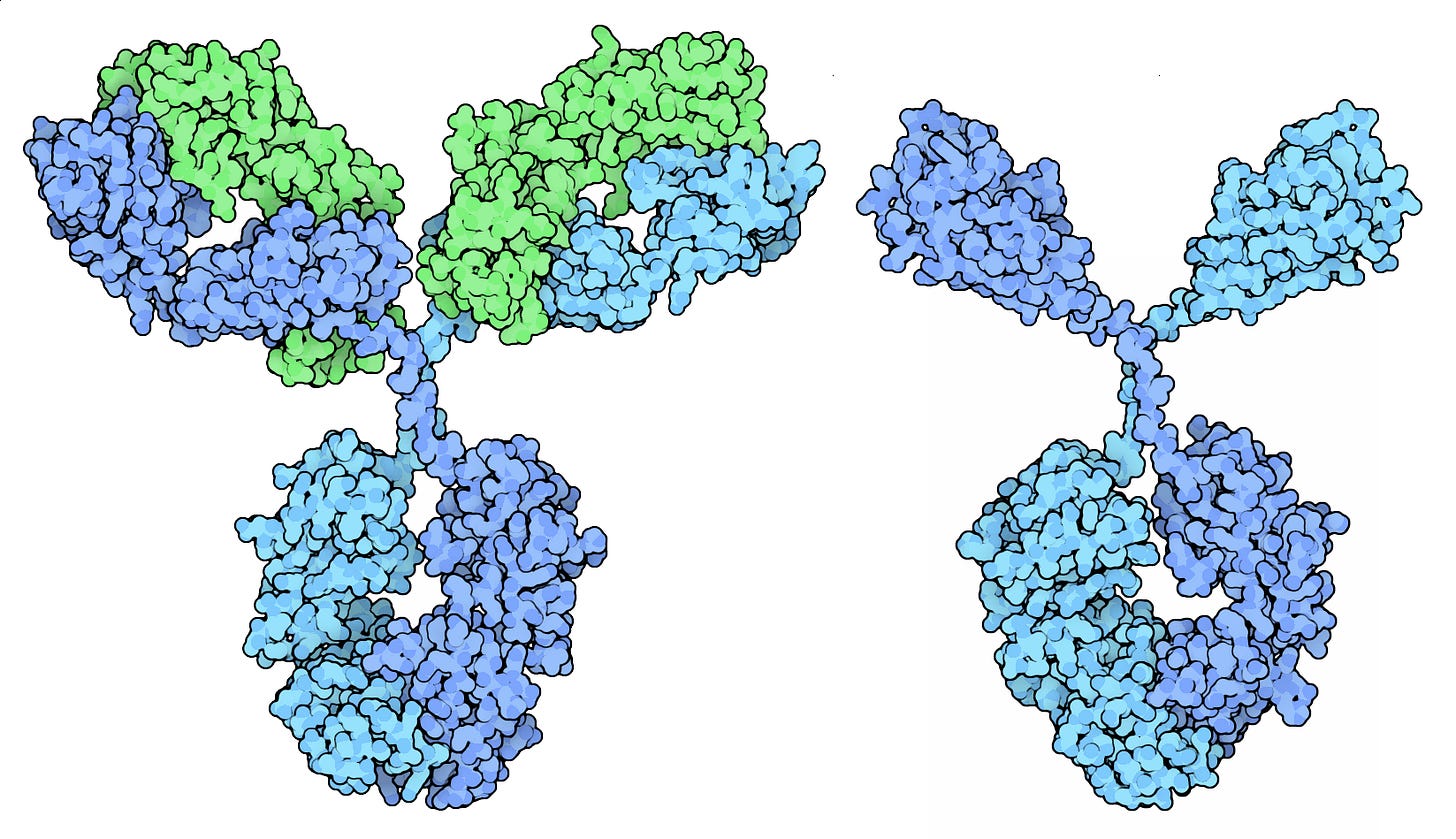
The first is that it may be more easily scalable, at least compared to the conventional production of antivenoms from horses and sheep. This has to do with this antivenom’s use of camelid antibodies. Historically, one of the reasons antivenoms were produced in horses was because they produce conventional antibodies (IgGs) that have a standard Y-shaped structure with two heavy chains and two light chains, resulting in large, complex molecules (approximately 150 kDa) that more closely mirror our own.
Camelids (camels, llamas, alpacas), in contrast, uniquely produce both conventional antibodies and a special type that lack light chains entirely, called “nanobodies.” These nanobodies are only about 15 kDa in size, or roughly one-tenth as large as conventional antibodies. Their small size allows them to reach venom molecules in hard-to-access areas of the body, and it was these nanobodies which were used to make the antivenom cocktail reported in this paper.
Nanobodies are also more stable, soluble, and less likely to trigger immune reactions in patients because of their simpler structure. And, finally, they are easier to manufacture than conventional antibodies because they are made from a single polypeptide chain. This structural simplicity means that they can be manufactured using microbes, rather than mammalian cells, which “might facilitate a lower cost of goods for treatment,” according to the paper.
While this approach already shows great promise, there are ways to optimize it further. This is especially true (and worth restating) because this biochemical approach is complementary to the work being done on AI-designed proteins.
In fact, Baker’s 2025 paper acknowledges that “RFdiffusion could be used to generate designs that neutralize other medically relevant toxins, expediting the formulation of antivenoms with broader species coverage.” When this work is expanded, researchers may be able to readily mix and match, manufacturing each nanobody in E. coli and combining them in desired ratios to confer even broader protection against snake venoms. An eight-protein cocktail, after all, is not the end-all be-all of broad antivenoms; these cocktails could expand to include AI-generated versions that cover that 18th snake, or that resolve some of the other envenoming symptoms observed in the mice in this study.
There is also the question of whether this approach can work elsewhere. While the paper demonstrates protection against African elapid venoms, more work is needed to see whether, for example, this approach could work in India, where 50,000 to 60,000 people a year die from snakebites.
In India, a group of snakes known as “The Big Four” — the Indian cobra, common krait, Russell’s viper, and saw-scaled viper — cause most of the deaths. The big four are split between elapids and vipers, resulting in a different venom composition. Whereas elapids contain the aforementioned toxin families (3FTx, PLA₂, and KUNs), viper venoms are predominantly composed of enzymatic proteins, such as snake venom metalloproteinases (SVMPs), snake venom serine proteases (SVSPs), and L-amino acid oxidases (LAAOs). These proteins attack blood vessels, prevent clotting, and destroy tissue. Protecting against these symptoms would require a different approach.
Finally, there is the longstanding question of who will pay for the development and manufacture of antivenoms, a question that has seen lots of recent handwringing as people consider what it would take to meet the WHO’s plan to halve the number of deaths and disabilities due to snake bites in South-East Asia by 2030. To revisit Bonde:
The field remains poorly funded. Investment into venomics research makes up just two percent of the snakebite budget in the WHO’s 2030 plan. Relying on venture capital instead, start-ups in the field are urged to focus on richer markets with high profit margins rather than where their products would do the most good. This is not to point blame at venture capitalists who are providing much-needed funding…but to emphasize that nonprofit funding in the space could shift priorities for the better.
To return for a minute, to my family’s llamas. When one was stuck in the pond, there was little one could do to encourage it to exit on its own. Instead, we had to wade in and haul it out. The same seems true of better antivenoms. Basic biochemistry or computational modeling might make them broad, but without our working to manufacture them at scale and haul them into the field, they will not arrive there on their own. As venomic researchers issue plaintive calls to philanthropists and discuss funding mechanisms like advanced market commitments, it would be remiss not to emphasize that innovation cannot carry a technology to widespread adoption. Scaling requires both buy-in and investment.
Xander Balwit is editor-in-chief of Asimov Press and recently changed her estimation of llamas.
Cite: Balwit, X. “An Antivenom Cocktail, Made by a Llama.” Asimov Press (2025). https://doi.org/10.62211/29kf-44ty
A huge thanks to Niko McCarty for directing me to this paper, talking me through it, and sending various reading materials in support. Thanks also to Ella Watkins-Dulaney for additional feedback and for creating the header image.
The use of camelids (llamas, alpacas, and camels) is not the groundbreaking part of this research. These animals have been employed in antivenom research and production since the late 1990s, when scientists first recognized that their unique antibodies — called nanobodies or VHH fragments — were stable alternatives to conventional antibodies derived from horses.
I mean, in no way, to diminish the value of this or other extant antivenom. According to the WHO, these antivenoms “can be highly effective, reducing mortality due to snakebite envenoming to less than 2 percent,” in sub-Saharan Africa. The Inoserp PAN-AFRICA antivenom is produced by the spanish biotech company, Inosan Biopharma. It is a polyvalent treatment designed to protect against African elapid and viper species and is made using horses. The antibodies are then purified from horse plasma and enzymatically digested to produce F(ab′)₂ fragments which are then filtered and freeze-dried into a powder that can be reconstituted for injection.
The speed at which these toxins spread depends on several factors: the location of the bite (bites closer to the heart or in areas with rich blood supply spread faster), the amount of venom injected, the type of snake, and one’s activity level — as movement and elevated heart rate accelerate the circulation of the toxins throughout the body.
I was looking for a way to shoehorn in some reference to my favorite Western novel of all time, True Grit, which ends with a race against time to save protagonist Maddie Ross after she is bitten by a rattlesnake. Now that I’ve done so, I’ll take this chance to recommend the movie “Mud” in which something similar transpires but with a cottonmouth. It seems I have a predilection for dramas with young children almost dying from snakebites if not for the heroic intervention of someone on the grey side of the law.
Elapid venom usually acts within minutes and peaks within a few hours, but its physiological effects can last one to two days. When antivenom is administered early, doctors know it’s working within the first couple of hours, but patients are kept under observation for at least 24 hours (often up to 48) to monitor neurotoxic symptoms, paralysis, and respiratory effects.
Apparently a bit of a misnomer, given that this snake slinks around in trees where it camouflages from predators, avoids people, and engages in ambush predation.


I am not a biologist but this was a fascinating read. Until now I was only aware that studying proteins can help in finding cure for diseases.
Good morning friend, I hope you are well.
I just wanted to drop a comment showing my appreciation for your work, it often appears on my feed and I enjoy it.
I collect historic books, I have 22 so far, 1707-1822, I thought my writings would be of interest to you, I base all my articles on historic books Alexandra:
https://open.substack.com/pub/jordannuttall/p/where-is-god?r=4f55i2&utm_medium=ios