What Makes an Experiment Beautiful?
A beautiful experiment is not just a reflection of human ingenuity but also efficient science.
Nature, mysterious in day’s clear light,
lets none remove her veil,
and what she won’t discover to your understanding
you can’t extort from her with levers and with screws.1Goethe, Faust I, lines 672–675
Only about the size of a pinhead, the early amphibian embryo undergoes rapid cell divisions immediately after fertilization. By the time this cellular sphere comprises a few thousand cells, the embryo begins to fold inward. The point at which it does so marks the dimple-like dorsal lip of the blastopore — the opening of the embryo’s newly forming central cavity. In the early 1920s, Hilde Pröscholdt, a talented graduate student under the supervision of Hans Spemann at the Zoological Institute in Freiburg, Germany, had observed this “gastrulation” process hundreds of times under her microscope.
Spemann’s own earlier experiments identified that the dorsal lip might be necessary for the embryo’s development, but how exactly it exerted its influence was unknown. Hilde Pröscholdt developed a delicate surgical technique that allowed her to excise the dorsal lip from the developing embryo of one newt species (Triturus cristatus) with unpigmented cells and transplant it into the same-stage embryo of another newt species (Triturus taeniatus) with brown cells, opposite its native dorsal lip. Thanks to the color difference between the host and the graft tissue, Pröscholdt was able to follow their fates.
It wasn’t until after numerous attempts,2 hampered by lack of antibiotics to ward off infections and the delicate nature of the surgery itself, that Pröscholdt observed a most marvelous result. By transplanting the dorsal lip from one blastopore onto a second embryo, she was able to coax the recipient embryo to grow into a two-headed organism, a pair of conjoined twins.3
Judging from the brown coloration of the twin embryo, the transplanted dorsal lip instructed the host cells to develop a second, complete body axis. The transplant’s own cells contributed only to the second notochord (a precursor to the spine in vertebrates). Spemann thus named the dorsal lip “the organizer,” as it instructed the fate of surrounding cells, steering and organizing their development into specific organs and tissues.4
Hilde Pröscholdt (Mangold after marriage) didn’t live5 to share the Nobel Prize in Physiology and Medicine that Hans Spemann received in 1935, but the discovery of the Spemann-Mangold organizer has repeatedly been called one of the most beautiful experiments in embryology. A Cells and Development editorial celebrating the centennial of this experiment in 2024 notes:
The exquisite beauty of the skillful experiments, histological sections, and written protocols of Hilde Mangold now form part of the history of biology. Her dedication to detail and excellence in this thesis for Freiburg University is probably without parallel.
But what does it mean for an experiment to be beautiful?
While every practicing scientist has an intuitive sense of what a beautiful experiment is, these features are rarely put into words. Nor is the answer static, and like aesthetic values more generally, these features changed throughout scientific history. In the 17th and 18th centuries, aesthetic appreciation of experiments was centered on nature unveiling its innate beauty. The principal job of a natural philosopher was to “reveal” what was already there.
But in contemporary terms, beautiful experiments are those that are distinguished by the aptness of their conception and execution (that is, how fit they are for the purpose of testing a particular hypothesis), by their clarity, ingenuity, simplicity of means, and by the importance of their results. Indeed, beautiful experiments exhibit a strong information asymmetry between the input from the experimenter and the output of the system under study. In the simpler words of the theoretical physicist and Nobel Prize laureate Frank Wilczek, in a beautiful experiment, “you get out more than you put in.”6
Amazingly, these features not only make experiments aesthetically appealing but also practically valuable. They are overrepresented among the historically decisive experiments that resolved major theoretical uncertainties in biology (and in the natural sciences in general). And while it may seem that a beautiful experiment results from a stroke of genius, perhaps we can invite more elegant experiments as a regular part of scientific practice. As a first step toward learning to design beautiful experiments, we need to better understand what makes them so.
Two Modes of Aesthetic Appreciation
During the Enlightenment in 18th-century Europe, experimental science was primarily seen as a means of revealing the beauty of nature itself. There was a strong belief in nature as well-ordered and comprehensible by the human mind, and its beauty as originating in God’s design.7 The Irish-Scottish philosopher Francis Hutcheson (1694–1746) even suggested that God had arranged this mystifying correspondence between the truths of nature and our experience of aesthetic pleasure and appreciation to encourage human curiosity.
An experimenter’s job was thus to expose the underlying forces that generated a variety of readily observable effects, akin to the unifying principle of least action: patterns of sound vibrations (Chladni plates), the dizzying variety of life forms, the symmetries of snowflakes and crystals (Haüy’s “primitive forms”). If nature were a painting, the experiment was but a frame to showcase it.
Rather than being a means for testing a pre-existing theory, the experiment was the starting point of an investigation in 18th-century natural philosophy. The primacy of experiment over theory was also related to the fact that scientific inquiry was essentially inductive: knowledge was (in the spirit of the philosopher Francis Bacon) built by amassing well-made observations and demonstrations and only then distilling them into theories.8
Experiments made in this tradition focused on revealing nature’s inherent beauty while the details of experimental design remained secondary. In the 1740s, for example, Abraham Trembley, a scholar and tutor from Geneva, chanced upon a strange creature in a sample of pond water. Though he wasn’t aware of it, some decades earlier, Antoni van Leeuwenhoek had identified this creature as a “water plant,” or a polyp. But upon closer inspection, Trembley observed that it had, instead, the habits of an animal — it could move, extend and contract its body, and prey on food with its mouth tentacles.
Trembley cut the polyps in half (and in many other ways) and watched in amazement as each fragment regenerated into a whole organism. He explored variations of this captivating process and extensively documented the outcomes. Most of his experiments were conducted in the palm of his hand with a pair of scissors, sometimes aided by a weak magnifying glass. In his 1741 letter to the French naturalist Réaumur, Trembley described these budding polyps as Hydre, and Réaumur excitedly shared his findings with the French Academy of Sciences.9
Trembley himself was hesitant to theorize about the significance of his discoveries. He eventually summarized them in a 1744 treatise, Memoir on the History of Polyps. Expressions of surprise, awe, and wonder permeate his writings, alongside meticulous descriptions and illustrations. His focus — and the focus of the scientific community that enthusiastically received his findings — was on the phenomenon of regeneration itself, with the experiments merely reflecting its careful study, only later distilled into theory by Trembley’s successors.10
The late 18th and 19th centuries, however, brought about a major shift in scientific methodology. With the development of the German research university model, where teaching and research went hand in hand, laboratories became the engines of knowledge production. Scientific equipment for observation and measurement became more standardized, supporting the emerging ideal of “mechanical objectivity.” (For one, photography started replacing artistic drawings of biological samples.)
In 1840, the English polymath William Whewell articulated the changing relationship between hypotheses and experiments that had begun to dominate in this new research environment:
The hypotheses which we accept ought to explain phenomena which we have observed. But they ought to do more than this: our hypotheses ought to foretell phenomena which have not yet been observed.
This sentiment was even more forcefully expressed by the French physiologist Claude Bernard in his 1865 treatise, Introduction to the Study of Experimental Medicine:
A hypothesis is … the obligatory starting point of all experimental reasoning. Without it no investigation would be possible, and one would learn nothing: one could only pile up barren observations. To experiment without a preconceived idea is to wander aimlessly.
The experiment now served to verify and illustrate (or refute the correctness of) preconceived theories, themselves building on earlier research.11 Scientific reasoning became more deductive, following from hypothesized generalities to observed particulars.
With this shift, there was also a change in how experiments were aesthetically appreciated. The attention switched to how the experiment was designed (how fit it was for the job of testing a hypothesis), rather than on what the experiment showed (the natural phenomena themselves). The beauty on display in the experiment was now that of human ingenuity and economy of experimental operations, rather than the underlying perfection of nature.
Louis Pasteur’s refutation of the theory of “spontaneous generation” is an elegant example of 19th-century experimental design coming to the fore. Many of Pasteur’s predecessors and contemporaries were proponents of spontaneous generation of life from a nutrient-rich source (like broth or meat) in contact with air. Pasteur’s hypothesis was, in contrast, that living microorganisms can only originate from pre-existing germs carried by the air, but not from the air itself.
To test this hypothesis, he commissioned special glassware for his lab in the form of a swan-neck flask with a round bottom. He carefully poured a nutrient-rich broth into the flask, then boiled it to kill germs present in the broth, and left the flask open to the air. Owing to the geometry of the flask, the air could freely contact the broth, but dustmotes carrying germs became trapped in the flask neck’s bends. Pasteur repeatedly observed that the broth would remain clear for a long time, under a variety of conditions — different weather, altitude, or geographic location.
But when he tilted the flask so that the liquid washed the trapped dust down to the bottom of the flask, or when he snapped the neck of the flask, the broth would soon become cloudy, indicating microbial growth. The observed phenomenon itself is arguably not spectacular in this case. However, the scientific community at the time appreciated and rewarded12 the ingenious experimental design, which was able to decisively resolve a centuries-long scientific controversy.
In the words of the physicist John Tyndall, who closely followed this debate, “there is no inference of experimental science more certain than this one.” And Pasteur, for his part, harbored no false modesty about his own achievement: “Never will the doctrine of spontaneous generation recover from the mortal blow of this simple experiment.”

Beauty in Experimental Design
For contemporary scientists, as inheritors of this 19th-century outlook, the focus on experimental design itself is all too familiar. But what exactly does it mean for the design of an experiment to be beautiful?
A beautiful experiment is more than just a good experiment — one that has appropriate controls, well-calibrated equipment, and clear reasoning from data to its interpretation. Whereas a good experiment is “merely” a trustworthy one, a beautiful experiment has additional qualities on top of trustworthiness, which is taken as an epistemic baseline. Such additional qualities have been suggested by both practicing scientists and philosophers of science to include economy and aptness of experimental design, clarity, decisiveness, ingenuity, simplicity of means, and significance of results.13
To return to Wilczek’s observation that in a beautiful experiment, “you get out more than you put in,” a beautiful experiment is thus also the opposite of brute force.14 It interrogates nature by a precise perturbation of a system that produces a rich and revealing response. Making such a delicate yet compelling perturbation requires the researcher to already have a good model of the system, noticing or stipulating some patterns that can be used to compress or skip unnecessary steps. Brute force methods, in contrast, often indicate a lack of a model. Interventions based on it are crude and seize on the system’s most superficial aspects.15
We can now see why the Spemann-Mangold experiment is considered one of the most beautiful experiments in embryology and all of biology. It not only demonstrates this notion of delicate perturbation and positive asymmetry of effort but also all the additional hallmarks of a beautiful experiment. It is conceptually simple: excise a patch of cells from one embryo and graft it onto another — crucially, while being able to visually distinguish the donor and recipient tissues.
But the Spemann-Mangold experiment was also based on a strong prediction about the unique role of the grafted tissue, the dorsal lip of the blastopore. And the embryo, being the very complex and sensitive system that it is, responds to grafting by massively restructuring itself in a revealing way, confirming the organizer hypothesis. This is one of the most significant results in embryology, and it also proved to be extraordinarily generative, opening up a whole subfield of embryonic induction, still actively researched a century later.
Simplicity comes up as one of the most frequently named qualities of a beautiful experiment. It represents a consistent, “classical” aesthetic value among scientists from the 19th century onwards. This includes both conceptual simplicity, simplicity of means (equipment and procedures), and operational simplicity (few sequential steps).
Another example of such simplicity can be found in the famous polyU experiment published in 1961 by Marshall Nirenberg and Heinrich Matthaei. A few years earlier, Francis Crick postulated that RNA may be the intermediate in the information flow from DNA to proteins. The discovery of the short-lived messenger RNA had been published earlier in 1961. In a beautiful experiment, Nirenberg and Matthaei showed that a cell-free extract of E. coli could be used as a model to determine if an RNA sequence could, in principle, program protein synthesis.
To simplify their experimental setup, the duo used synthetic RNA made only of uridine nucleotides (polyU). To the cell-free extract with polyU, they added a mix of all twenty canonical amino acids, while radioactively labeling only one of them at a time. Only when phenylalanine was labeled were they able to isolate a polypeptide made entirely of that amino acid, indicating that some number of U nucleotides encodes it (the triplet nature of code wasn’t revealed until later in another set of elegant experiments by Francis Crick and colleagues).
Beyond its simplicity of design, the beauty of the polyU experiment lies in the clarity of its results: a one-letter RNA template collapses an immense search space (all possible nucleotide sequences of a given length) into a clear readout — a single specific polypeptide, and the assay itself is aptly designed to answer the core experimental question: is there a correspondence between an RNA sequence and a specific amino acid and, if so, what is it in the case of polyU? As a first step toward cracking the genetic code, the importance of this experiment cannot be overstated.
Closely related to clarity of results is decisiveness, another feature of a beautiful experiment. It also defines “crucial experiments,”16 those that conclusively rule out between competing hypotheses. Such experiments help us attain a sense of completeness in understanding a particular phenomenon.
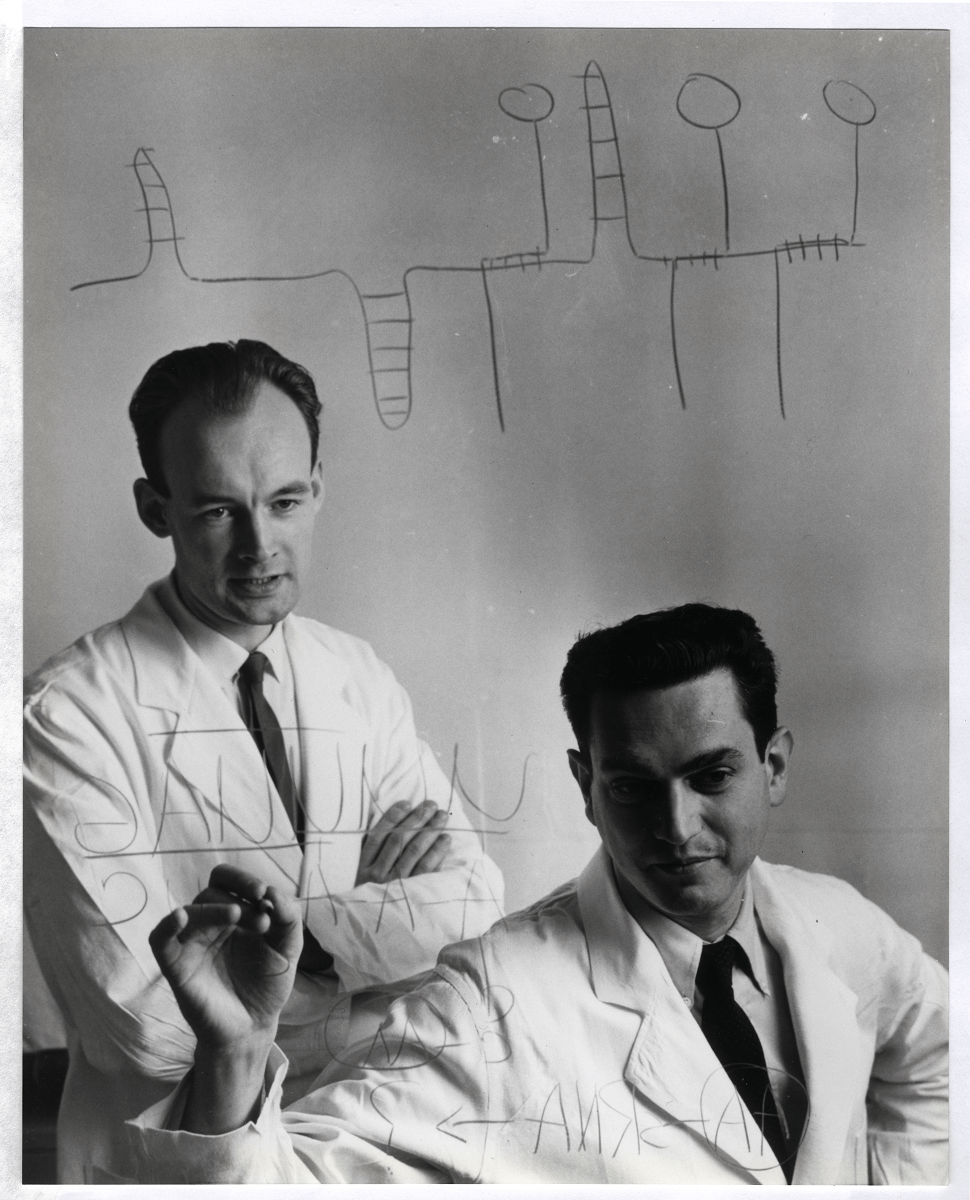
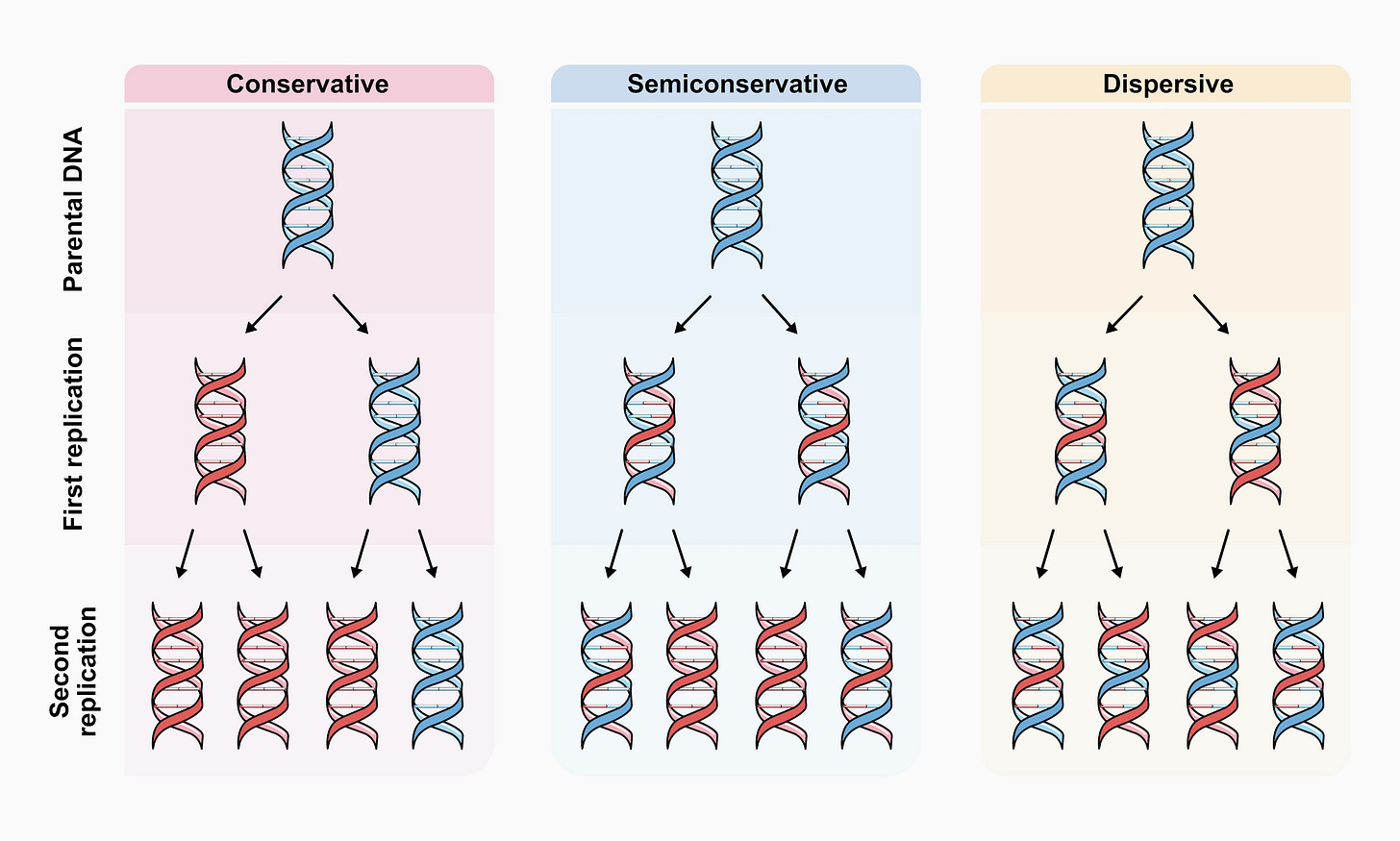
Perhaps the best example of this is the Meselson-Stahl experiment, dubbed by the British physician and biochemist John Cairns “the most beautiful experiment in biology.” This experiment decisively established the semiconservative mechanism of DNA replication while ruling out the two other alternatives, conservative and dispersive replication.
In their seminal publication of the structure of DNA in 1953, James Watson and Francis Crick suggested how DNA may replicate, though they initially did not articulate this process clearly in print. This became known as the semiconservative model, where the two DNA strands separate during replication, each then serving as a template for the synthesis of a daughter DNA molecule. But there were doubts among molecular biologists at the time as to whether it is energetically possible for the DNA strands to separate in this way — that is, if the hydrogen bonds between the DNA bases could be “unzipped” along the entire length of the molecule.
As an alternative, Gunther Stent, professor of molecular biology at the University of California, Berkeley, proposed the conservative replication model, where both strands of the parental DNA molecule were copied without breaking it, so that, at the end of replication, there remained the intact parental molecule and a full, newly synthesized one. In contrast, Max Delbrück, founder of the Phage Group at California Institute of Technology, came up with the dispersive model, where DNA was synthesized in short sequences alternately from both strands, by breaking them at intervals. In this model, parental fragments were interspersed with newly synthesized ones in the daughter molecules.
Matthew Meselson and Franklin Stahl realized that these models “differ in the predictions they make concerning the distribution among progeny molecules of atoms derived from parental molecules.” The ingenuity of their experiment was in the labeling technique that they used to follow the fates of the DNA strands and the ability to separate them by weight using ultracentrifugation.
The two researchers used a heavy nitrogen isotope, 15N, to label DNA molecules in the bacterium E. coli by growing the bacterial cultures in a medium containing ammonium chloride (NH4Cl) as the only source of nitrogen (which the bacteria incorporated into DNA bases through metabolism). The bacteria were allowed to grow in the heavy nitrogen medium for long enough — 14 generations — so that all of the nitrogen atoms in their DNA could be reasonably expected to be the heavy isotope.
Then, Meselson and Stahl abruptly changed the nitrogen source to ammonium chloride containing the light isotope of nitrogen, 14N. By isolating DNA from the subsequent generations of E. coli grown on light nitrogen and running them in the ultracentrifuge,17 they showed that the first generation after the isotope switch had an intermediate density compared to DNA fully labeled with only the heavy or only the light isotope. In the following generations, the DNA partitioned between the intermediate and light fractions, exactly as predicted by the semiconservative model.18
The Meselson-Stahl experiment was immediately recognized by the scientific community as “extremely beautiful,” and Meselson was even christened by Gunther Stent as “the Mozart of molecular biology.”
In his book Beauty and the Beast: The Aesthetic Moment in Science, the German historian of science Ernst Peter Fischer aptly remarked on the conceptual beauty of the Meselson-Stahl experiment:
One condition of this experiment consisted in making the genetic material physically heavier without changing it chemically. There is something beautiful in this idea alone, the understanding that the chemical properties of an atom — for example, its ability to bond with other atoms — are determined by its external electrons, whereas the physical properties — for example, the mass — are hidden inside the atomic nucleus.
Like Spemann-Mangold, the Meselson-Stahl experiment thus embodies the full range of features of a beautiful experiment: simplicity of concept and execution, clarity, unequivocal interpretation (decisiveness), ingenuity, and significance of the outcome.
The Aesthetics of Contemporary Biology
Most experiments in biology that are historically recognized as beautiful come from the 20th century, when the field came of age as a quantitative science. This period was when cellular and molecular levels of resolution became accessible thanks to advances in microscopy, spectroscopy, and crystallography. Since the scope of such experiments was usually limited to one gene, one biochemical reaction, or a specific cellular or physiological context, a minimal experimental setup could settle a major theoretical question. It was feasible to appreciate the significance of the results almost immediately, by a clear visual readout, as in the case of the polyU and Meselson-Stahl experiments.
In contrast, 21st-century biology is a science of large-scale experiments that lack this observational immediacy and require lengthy computational data analysis to interpret. With expanding technological advances, we can now analyze whole genomes, transcriptomes, proteomes, and metabolomes of thousands of cells at a time; however, it’s rarer for such experiments to meet the criteria of “classical” beauty, as seen in the earlier history of biology. Instead, their beauty resides at a more abstract, conceptual level rather than in their direct execution, which is often more complex with multiple steps.
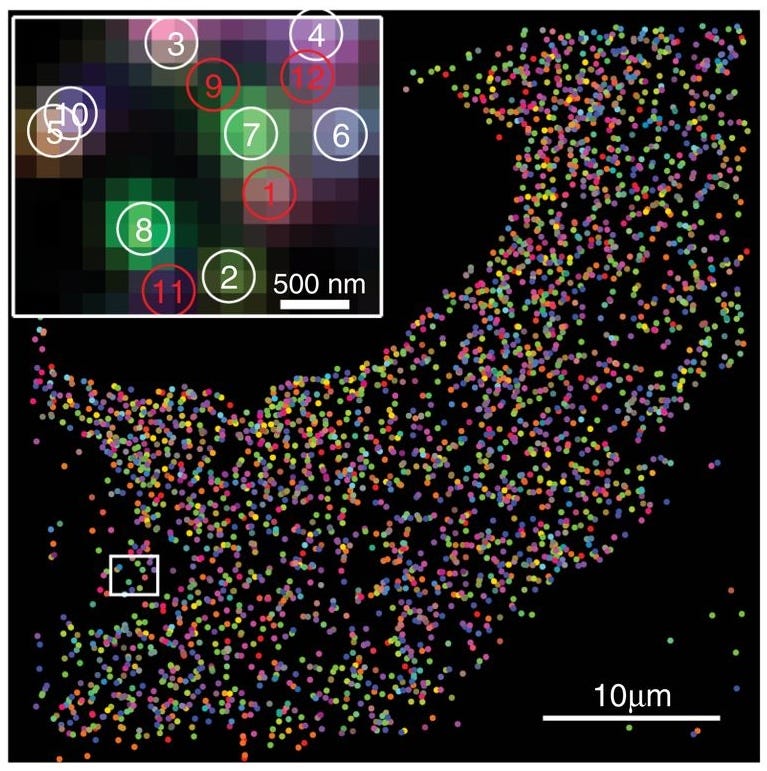
One exemplar of the abstract beauty of contemporary biology is MEMOIR (memory by engineered mutagenesis with optical in situ readout), a system for cell lineage tracing developed by Michael B. Elowitz, Long Cai and their colleagues at the California Institute of Technology. MEMOIR is essentially a way of tracing the fates of individual embryonic cells to map their lineage and the location of their descendants in the body.
In mice, for example, the method requires the following steps. Researchers first engineer embryonic stem cells to carry two key components: an inducible Cas9 gene and guide RNAs that point the Cas9 protein to specific DNA sites in the genome, called “barcoded scratchpads.” Each scratchpad contains a short DNA repeat sequence followed by a unique identifier, or barcode.
When the Cas9 gene is activated,19 it makes the gene-editing protein, which grabs onto the guide RNAs and then cuts the scratchpads, occasionally deleting portions of them — a process the authors call scratchpad “collapse.” Over successive rounds of cell division, different cells randomly accumulate distinct patterns of collapsed and intact scratchpads, thus producing a combinatorial record of their shared and divergent histories.
The scratchpads of each cell in the developed organism can then be read out using a spatial sequencing technique, such as single-cell fluorescent in situ hybridization (smFISH), which detects mRNA synthesized from the scratchpads, without breaking up the cells, in their native context. The end result of this work is that scientists can figure out which cells came from which progenitors and where those cells reside in the organism. It is developmental biology at the scale of organisms, but with the resolution of single cells.
MEMOIR is beautiful, in part, because its inputs are sparse: Cas9 activates in a time-sensitive manner and introduces a molecular record of its activity, which then carries over to progenitor cells. The output, however, is tremendously rich and previously technologically unattainable: reconstruction of a full cellular lineage. Arguably, the technical execution of the MEMOIR experiments requires multiple steps of genome engineering, smFISH, imaging, and image analysis — all far from simple. Yet, conceptually, it is a beautiful and elegant experiment (or rather, an experimental method).
Aptness of design and conceptual (if not operational) simplicity thus remain hallmarks of a beautiful experiment in 21st-century biology, even if the experiments in general have become technically much more complex.
And while Enlightenment era thinkers believed that nature was beautiful in its simplicity and comprehensibility, we now know that nature (the totality of all living things) is a product of the blind and stumbling evolutionary process and doesn’t always arrive at the most beautiful and economic solutions. What matters is that its solutions are good enough for survival and reproduction. Therefore, biology need not abide by our expectations of beauty.
For example, Francis Crick’s initial elegant idea of a unique triplet of bases for each of the twenty amino acids proved to be erroneous — it was later established that, after all, most amino acids are encoded by more than one triplet of nucleotides. In the words of William Newsome, the co-chair of the BRAIN Initiative, “In biology, it is possible to be elegant and be wrong.”
Still, it can be argued that while biology itself and our theories about how it works indeed need not always be beautiful, there’s a lasting value in designing our experiments beautifully. That is because experiments are our tools for interrogating nature, and we may as well sharpen those tools by making them most apt for the purpose. In principle, it is possible to design a beautiful experiment even if our object of study is messy and complicated. What matters is how we ask our questions and how we poke and prod the system under study to “persuade” it to reveal its workings to us.
But of course, the everyday practice of science doesn’t proceed in a series of neatly designed experiments that work on the first try. Indeed, practicing scientists have spoken about the messiness, uncertainty, and stumblings in both conceiving and performing experiments. A beautiful experiment may in fact be a distillation of many months of such messy “night science” (a coinage by the French biochemist and Nobel laureate François Jacob).20
Still, the kind of “workshop” that night science grants us may be necessary to get “a feeling for the organism,” as another Nobel laureate, Barbara McClintock, said of the attunement to maize that she developed during her lifelong work on mobile genetic elements in this plant. Only with such an attunement in place can one develop good models of a system that enable the design of beautiful experiments.
Ultimately, as our exploration of beautiful experiments has shown, such experiments are not only good and trustworthy but also apt, simple (at least conceptually), economical, and revelatory. They are exceptionally well-suited for the purpose of testing a particular hypothesis; they contain the necessary steps and nothing extraneous, and yield high informational gain. Whatever our object of study, approaching it with an experimental design that has these properties moves beyond mere aesthetic appreciation into the realm of the most fruitful and fortuitous science.
Ulkar Aghayeva is a science writer and a columnist at Asimov Press. She also writes about music history and cognition at The Bass Line.
Cite: Aghayeva, Ulkar. “What Makes an Experiment Beautiful?” Asimov Press (2025). DOI: https://doi.org/10.62211/97qs-98pk
The author thanks Amanuel Sahilu, Jacob Kimmel, and Ella Watkins-Dulaney for helpful and stimulating discussions that contributed to this piece. Lead image by Ella Watkins-Dulaney.
Translation by Stuart Atkins.
In total, Pröscholdt carried out 259 transplantation experiments, of which five were presented in her 1924 publication with Spemann (and another two mentioned but not shown). Such a paper would likely have a hard time getting published in our time due to the low number of samples, but luckily it was well-received in its own time.
Pröscholdt carefully dissected these embryos and made a series of contrast stainings of thin cross-sections and detailed hand drawings based on them. This allowed her to follow the fates of both host and graft tissues at the cellular level, thanks to their distinct pigmentation.
Strictly speaking, a group of cells is defined as an “organizer,” in Spemann’s sense, if it is capable of inducing a neural plate (the precursor of the nervous system) and a complete body axis. Later research showed that the organizer is a source of signaling molecules, most of which inhibit major developmental pathways.
Her life ended tragically early at the age of 26, just before her paper with Spemann was published in 1924.
This quote is from his 2016 book A Beautiful Question: Finding Nature’s Deep Design.
Robert Hooke even regarded beauty as the distinguishing feature of natural phenomena as opposed to man-made artifacts: “So unaccurate is [Art], in all its productions, even in those which seem most neat, that if examin’d with an organ more acute than that by which they were made, the more we see of their shape, the less appearance will there be of their beauty: whereas in the works of Nature, the deepest Discoveries shew us the greatest Excellencies.”
As the Enlightenment chemist Joseph Priestley wrote, in 1779, we are “too much in haste to understand, as we think, the appearances that present themselves to us. If we could content ourselves with the bare knowledge of new facts, and suspend our judgement with respect to their causes, till, by their analogy we were led to the discovery of more facts, of a similar nature, we should be in a much surer way to the attainment of real knowledge.”
During a public experiment, he shouted, “Amazing! No one had ever seen animals reproducing with a method normally used to kill them.”
Trembley’s work lent support to proponents of epigenesis, who thought that the embryo develops gradually, in their ongoing debate with preformationists, who argued that the embryo forms from preexisting parts (the proverbial homunculus).
On this transition, the philosophers of science Glenn Parsons and Alexander Rueger note, “A famous and spectacular clash of the old and the new way of doing science occurred when Lavoisier, in 1789, started his textbook of the ‘new chemistry’ by laying out the theoretical doctrine instead of beginning with a description of experiments and apparatus and then gesturing towards a theory only after the facts had been laid out.”
In 1862, Pasteur was awarded the Alhumbert Prize established by the French Academy of Sciences specifically for resolving the debate on spontaneous generation.
Though the latter criterion is a point of contention among philosophers of science. Not all agree that the significance of results is necessary to recognize an experiment as beautiful. Instead, the experimental design itself may suffice on its own terms, even if the experiment doesn’t lead to a groundbreaking discovery or else yields a null result.
There are, of course, also reasons to admire the sheer energy behind some of the brute force experiments in science history. The rivaling labs of Roger Guillemin and Andrew Schally processed millions of sheep and pig hypothalami in their quest to isolate milligrams of peptide hormones produced in that tiny part of the brain. Such work required deep conviction, relentless grit, and determination over years. But we wouldn’t call those experiments beautiful. In part, they were limited by the crude technology available at the time, and brute force was necessary.
I thank Amanuel Sahilu for pointing out this connection.
Experimentum crucis, or a crucial experiment, is a term coined by Robert Hooke and first applied by Robert Boyle to describe Blaise Pascal’s mercury barometer experiment in 1648.
The samples are placed in a solution of cesium chloride and rotated at high speed. The denser the material, the farther it travels away from the axis of rotation. The solution of cesium chloride forms a gradient of increasing density with increasing distance from the axis of rotation. The DNA samples reach equilibrium at the position where their density equals that of the solution.
In contrast, the conservative model predicts only light and heavy DNA in the next generations, while the dispersive model predicts only DNA of intermediate density in all subsequent generations.
To be precise, Cas9 is fused with a degron that targets it for continual degradation until a small molecule called Shield1 is added to prevent the degradation from happening, amounting to Cas9 activation.
Full quote from Francois Jacob, Of Flies, Mice, and Men and The Statue Within: An Autobiography (as quoted here):
Night science … hesitates, stumbles, recoils, sweats, wakes with a start. Doubting everything, it is forever trying to find itself, question itself, pull itself back together. Night science is a sort of workshop of the possible where what will become the building material of science is worked out…Where phenomena are still mere solitary events with no link between them … Where thought makes its way along meandering paths and twisting lanes, most often leading nowhere … What guides the mind, then, is not logic but instinct, intuition. The need to understand.

How beautiful
Some of the experiments included in this gallery have beautiful results — not just because the images they’ve captured are beautiful to look at. Great to see cutting edge science married to art like this (wonder is at the root of both science and art, ultimately).
https://unswscience.substack.com/p/the-secret-beauty-of-microscope-art?r=62e707