The Uncertain Origins of Aspirin
The history of humanity’s pharmacopeia is often muddied by folklore. What can the origins of aspirin teach us about separating fact from fiction?
Watch our behind-the-scenes interview with the author on YouTube.
By Sean Harrison
On a cool starlit night in ancient Egypt, a wife brews willow-bark tea for her husband, hot with fever;
While Athens and Sparta wage war, Hippocrates hurries to the river to shave some more willow bark, intending to cool down another sick child brought to his door;
While George III marries Princess Charlotte, a son dashes off into the woods in search of willow bark to treat his mother’s worsening fever.
While these anecdotes are familiar from cinema and literature, the history of aspirin and the willow bark from which it is (supposedly) derived is far from clear.
For instance, it is often said that Hippocrates prescribed willow bark tea to treat inflammatory pain. In a perfect world, we would know this because a verified scroll from around 400 BC would exist, stating: “For inflammation, I recommend willow bark tea, and so does everyone I know. So sayeth Hippocrates of Kos, future father of medicine and writer of the eponymous oath.”
Of course, such primary material does not always come to light. And many who recount medical and scientific histories (or, indeed, any history at all) do so despite incomplete information. In these cases, however, it’s still important to cite what evidence there is: what, for example, has led so many people to believe Hippocrates prescribed willow bark tea for inflammatory pain? If the answer is “someone said so,” who was that someone, and why did they think it was true?
This history of one of the world’s most widely consumed classes of drugs demonstrates the difficulties that surround the fact-finding of its origins. Some of these difficulties are historic, such as bad record keeping or obscure translations, and some are of continued relevance, such as political upheaval or the prejudice that often interferes with science.
My goal is not only to tell the story of a pharmaceutical staple but to share what my decades-long work in evidence synthesis in epidemiology has taught me about researching scientific history. In doing so, I’ll differentiate claims that are known from original evidence — such as the writings of Hippocrates and Pliny the Elder or original journal articles — from those made without primary support. I have sourced as much of the latter as possible, but both conflicting and unsupported claims remain, which I’ll note.
The origins of aspirin make a good subject for exposing the challenges of scientific research for a couple of reasons. Firstly, the stories stretch back millennia, and secondly, aspirin is such a successful drug that there is ample material to work with. Aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs)1 are among the most widely prescribed drugs worldwide,2 are safe enough that many are available without a prescription, and are some of the cheapest available drugs of any class. It’s often claimed that 30 million people take NSAIDs worldwide every day, although this assertion originally dates back to 1987 and wasn’t referenced. Nonetheless, if it were true, we would expect that number to be substantially higher now, given the increase in global population.
But before we can contend with the popularity of NSAIDs, we must first look at their origins.
Ancient History: Antiquity to 1763 AD
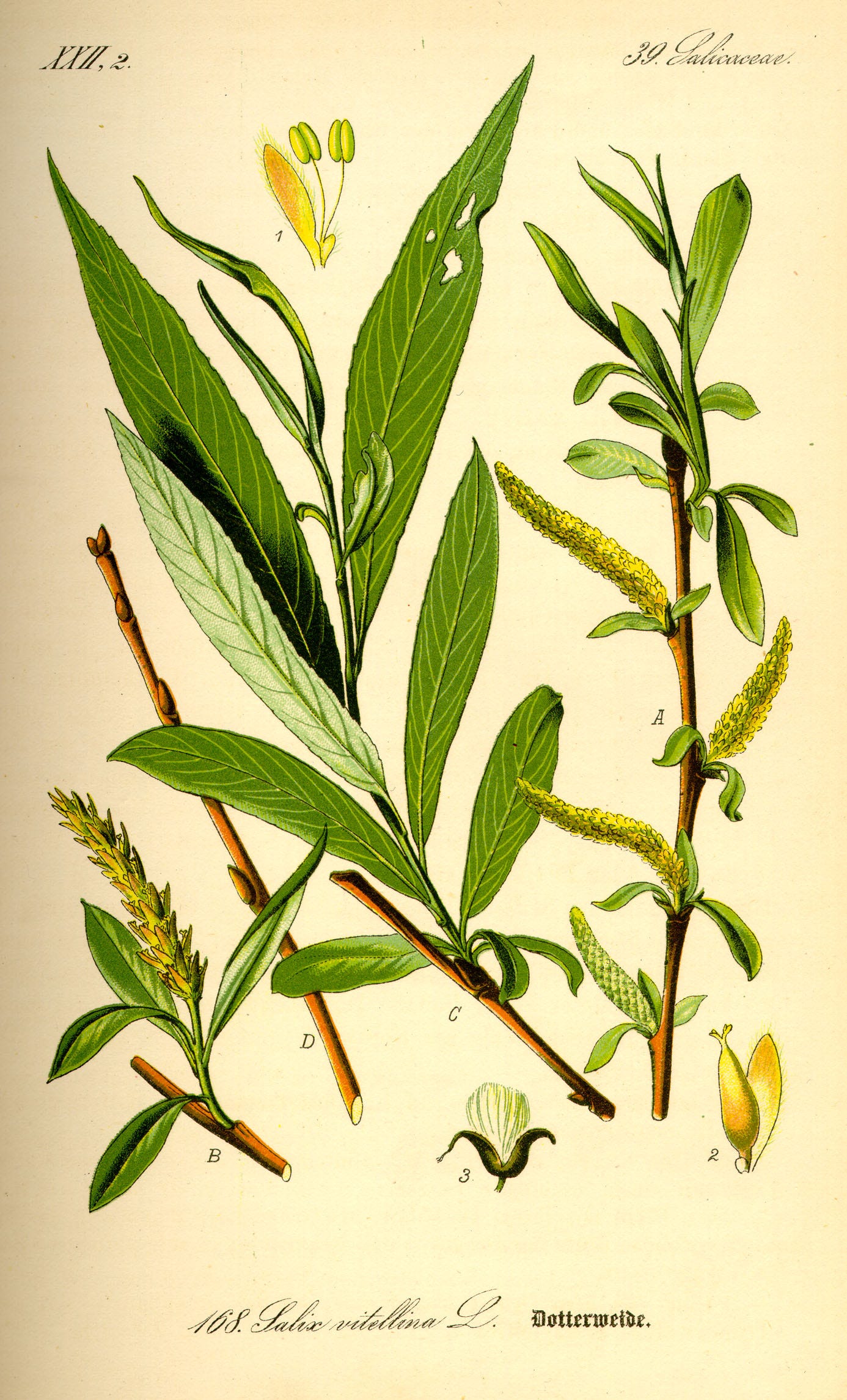
When I was in medical school in 2007, I learned that willow bark tea has been used for thousands of years to treat pain and fever. I’ve read at least a handful of books — historical and fantasy alike — that make the same claim. The fifth Bridgerton book, To Sir Philip, With Love, includes a scene with an urgent search for willow bark to bring down a character’s fever. Diarmuid Jeffreys wrote an entire book on aspirin, The Remarkable Story of a Wonder Drug, which detailed how willow was used from Ancient Egypt, through Ancient Greece and Rome, to the Middle Ages in Europe, and beyond. This is repeated by journal articles, though their claims are rarely referenced.
Even if their sources are sketchy, these accounts seem plausible. Willow trees are relatively common, grow worldwide (though mostly in colder and wetter parts of the Northern Hemisphere), and have had various uses since antiquity. For example, a willow fishing net was found in Finland dating from 8,300 BC, and willow has also been used for wattle fences, wattle and daub houses, coracles, cricket bats, and even World War II parachute baskets.

However, the most plausible reason that willow bark could be the antecedent of aspirin is that it contains salicin (named for the genus of willow trees, Salix). Salicin is converted into salicylic acid in the body when an enzyme bisects salicin, one half of which is then oxidized to become salicylic acid. Acetylsalicylic acid, known today as aspirin, is also converted into salicylic acid in the body: an enzyme simply cuts off the acetyl group to give salicylic acid. Salicylic acid is likely the active compound that blocks the enzyme cyclooxygenase, giving aspirin its efficacy, and was used as a treatment for fever and gout before aspirin was developed.
It seems reasonable, therefore, to believe that willow bark tea could act like aspirin, reducing fever, inflammation, and pain. Willow grew in abundance across the world, and people have been using it for thousands of years. Additionally, myrtle and poplar trees, as well as meadowsweet, likewise contain salicin and could have been used for the same purpose.
However, there is a problem with the claim that willow bark and similar plants are the ancient equivalent of aspirin when brewed as tea or simply chewed. And it is this: most of the extant evidence referenced by histories of aspirin fail entirely to mention using willow bark or similar plants to reduce fever, pain, or inflammation.
When people talk about evidence of the ancients brewing or chewing willow and similar plants, they often invoke the Ebers papyrus, Hippocrates, Celsus, Pliny the Elder, and Pedanius Dioscorides.3
The Ebers papyrus is an ancient Egyptian medical scroll from about 1550 BC, though it may have been copied from earlier texts. Translated by German Egyptologist Georg Ebers in 1875, the papyrus has 110 pages, is 20 meters long, and includes 700 remedies for various afflictions. As a piece of history, it’s fantastic. And some of the remedies are still practiced today: the treatment for Guinea-worm disease was, and still is, to wrap the emerging end of the worm around a stick and slowly pull it out.4
When I searched through a translation of the papyrus, however, I saw no evidence of willow bark used similarly to aspirin. I did find a treatment for an “ear-that-discharges-foul-smelling-matter” that used “berry-of-the-willow” and a remedy to “put the Heart into proper working order and make it take up nourishment” that used “Willow-tree, one-eighth part, added as a stiffening.”
There was also a remedy to make the “met” supple (nerves or possibly blood vessels, Egyptologists appear uncertain) that used “splinters-of-the-willow-tree,” although this particular remedy also required “hog’s dung” and “myrrh” among 34 other ingredients. Still, there was nothing about using willow bark specifically concerning pain, inflammation, or fever.
In his 1992 book Murder, Magic, and Medicine, author John Mann wrote that the Ebers papyrus contains the following remedy: “When you examine a man with an irregular wound … and that wound is inflamed … [there is] a concentration of heat; the lips of that wound are reddened and that man is hot in consequence … then you must make cooling substances for him to draw the heat out … [from the] leaves of the willow,” but I’ve been unable to find this in any translation of the papyrus, and Mann doesn’t reference the translation he claims to be using.
Uncertainty about the role of willow bark in treating inflammation remains as we move to Hippocrates, the ancient Greek physician and philosopher often regarded as the father of (clinical) medicine.5 Pharmacology lecturer Philippa Martyr suggests the works ascribed to Hippocrates only reference willow once, specifically in the context of burning willow leaves to make smoke for fumigating the uterus to get rid of a miscarried pregnancy. Indeed, a French translation of Hippocrates’s complete works confirms this. I was, however, unable to find any reference to willow in other translations. It is also said that Hippocrates recommended chewing willow bark to relieve fever and pain, but this, too, lacked evidence.

Other ancient sources are similarly unclear. The Roman encyclopedist Celsus, who wrote De Medicina in the first century, suggested willow leaves boiled in vinegar could be used to treat ulcerations of the anus and prolapse of the anus and uterus (I’m not sure whether that would work, and it certainly isn’t a common use of aspirin today).
Pliny the Elder, a first-century Roman author and natural philosopher, expounds the uses of poplar and willow trees in his encyclopedia Natural History. For poplar trees, he suggests, “A potion prepared from the bark is good for sciatica,” and “The leaves, boiled in vinegar, are applied topically for gout.” And for willow: “The leaves, too, boiled and beaten up with wax, are employed as a liniment for … gout.” This treatment is echoed by Pedanius Dioscorides, a Greek physician in the Roman army, also writing in the first century, who describes a similar treatment in his pharmacopeia, De materia medica. This is the earliest reference I have found to using salicin-containing plants as we’d potentially use aspirin today.
So ultimately, while we have a smattering of evidence that some version of a poplar bark potion may have been used for sciatica and that poplar and willow leaves may have been used for gout, we arrive at our second problem with the claim that willow bark and similar plants are the ancient equivalent of aspirin — that of effectiveness.
The amount of salicin in plants, even willow bark, is negligible compared to the aspirin dosages that we deem effective today. When researchers gave people willow bark extract corresponding to 240 mg of salicin, then looked at how much salicylic acid was present in their blood over time, it was the equivalent of taking 87 mg of aspirin (300 mg to 600 mg is recommended per dose, with up to 3600 mg allowed per day). Notably, 240 mg of salicin is the recommended daily dose specified by the European Scientific Cooperative on Phytotherapy.
But 240 mg of salicin today comes from concentrated and standardized willow bark extracts, not raw bark or leaves. The actual salicin concentration in willow bark is wildly variable, ranging between 0.04 percent and 12.06 percent across different species, according to one study. Salicin concentrations also vary across plants seasonally and based on a tree’s age. Only a fraction of the salicin would remain in tea or chewed bark.
If, for example, each cup of tea provided 240 mg salicin (possible with a good steeping and a high salicin content in the bark), then one would need to drink 41 cups of tea to get a full, therapeutic aspirin dose of 3600 mg. This is about 10 liters or two-and-a-half gallons.
Willow bark tea is also notoriously bitter, likely due to the high concentration of tannins, which can cause an upset stomach and nausea. Even if you could push through the bitterness, it’s unlikely you’d be able to stomach the bucketfuls of tea required to get enough salicin from willow bark (or similar plants) to ease your discomfort.
That’s not to say willow bark and similar plants couldn’t be effective in reducing pain, inflammation, and fever when steeped or chewed, however. Unlike aspirin, willow bark contains other compounds — flavonoids and polyphenols — that may contribute to alleviating such symptoms. However, few (if any) human studies have been conducted looking specifically at simply brewing or chewing these plants. While some research has been done on the efficacy of willow bark extract in the treatment of pain and inflammation, the extract is much more concentrated than in chewed bark, and results have varied.
Philippa Martyr adds an additional caveat when she notes:
If willow bark and leaves were handy and potent painkillers, we would have used them almost to extinction by now.
Overall, given patchy evidence and dubious efficacy, I am skeptical that the history of aspirin began in antiquity. The mythology of aspirin’s ancient history is, most likely, a coincidence: it’s easy to believe that because willow bark contains salicin, and it was possibly used for some of the same things as aspirin, and it was possibly effective, it must work, and therefore willow bark tea was ancient aspirin. It makes for a plausible story with historical continuity, but I believe it remains just that — a story.
For what I believe to be the most credible origins of aspirin and other NSAIDs, we must advance to 18th-century England.
Discovery of Aspirin: 1763 to 1877
The Reverend Edward Stone appears to have given the first published account of using willow bark extract to treat patients in Chipping Norton, England, in 1763. The patients were suffering from malarial fever (ague), which was still endemic to England at the time. He writes:
There is a bark of an Englifh tree, which I have found by experience to be a powerful aftringent, and very efficacious in curing aguifh [agues] and intermitting diforders.
Like many of his predecessors, Reverend Stone believed that the remedy to a malady could be found close to the cause. That is, if people were becoming sick in a certain place, there should be a plant or other remedy nearby.6 As willow trees grew in abundance in “moist and wet soil where agues chiefly abound,” and because it was as bitter as “Peruvian bark” (Chichona tree bark, which contains quinine, a traditional remedy for malaria), Reverend Stone thought he could use the dried, powdered bark to treat fevers.7

Drying the bark increases its potency, which gets around the issues of having to drink bucketfuls of tea — similar to using willow bark extract today. For three months, Stone dried a pound of bark next to a baker’s oven before pulverizing it into a powder. He started small, giving his first patient “twenty grains of powder” in “water, tea, small beer and such like,” but quickly increased the dose after noticing no side effects. He treated fifty people over five years, all of whom he said were either cured or helped by the treatment.
Notably, Stone had investigated whether his treatment had precedent, but came up empty-handed:
My curiofity prompted me to look into the difpenfatories and books of botany, and examine what they faid concerning it; but there it exifted only by name. I could not find, that it hath, or ever had, any place in pharmacy, or any fuch qualities, as I fufpected afcribed to it by the botanifts.
Stone’s observation of the novelty of his treatment is corroborated by the medicinal herbalist Anne Stobart, who looked through 6,500 17th-century medicinal recipes for 40 plants with longstanding use in the UK, determining that willow had six or fewer mentions.8 It seems fair to conclude that, at least in the UK in the 17th and 18th centuries, willow was not a well-known treatment for pain, fever, or inflammation. Stone documented his use of it, but it was by no means a widespread curative.
Around 1824, about 50 years after Stone’s investigation of willow bark powder, Italian pharmacist Bartolomeo Rigatelli extracted a “very bitter antipyretic” called “saline” from a plant native to Europe. The “saline” was subsequently renamed “salicin” by Francesco Fontana, who also extracted it from white willow and used it as a substitute for quinine sulfate. Fontana reported it was effective against fevers of all types, even “quartan fevers” (one of the four types of malaria). And while Fontana did not reference Rigatelli, he did reference someone named “Stone.” While there’s no way to be certain, it seems likely this was Reverend Stone.
The progression from salicin to aspirin would take over 70 years. In 1829, French chemist Henri Leroux refined the process used by Rigatelli, Fontana, and others and managed to extract pure salicin. His method was further refined in 1838 when Raffaele Pirìa, an Italian chemist, produced salicylic acid from salicin, after determining its molecular formula. By 1859, the German chemist Hermann Kolbe used the discovery of salicin’s molecular structure to synthesize salicylic acid directly. Kolbe’s process was refined by his assistant, Rudolf Wilhelm Schmitt, with the resulting process known as the Kolbe-Schmitt Reaction.9
In the 1870s, salicylic acid took off as a treatment, and in 1874, Friedrich von Heyden, another student of Kolbe, opened a factory in Radebeul (near Dresden) to produce it, reportedly selling it ten times more cheaply than its naturally derived counterpart. This spurred research into its clinical applications. In 1876, a Scottish physician, Thomas MacLagan, and a German physician, Franz Stricker, both published studies showing that salicin and salicylic acid were effective in treating rheumatic fever, particularly in reducing fever and pain. A year later, in 1877, a French physician, Germain Sée, showed that chronic rheumatism and gout could also be treated with salicylic acid.
Salicylic acid had proven effective in reducing pain, fever, and inflammation, and it could be manufactured cheaply and in large quantities. However, it had several serious side effects, such as nausea, gastric irritation, and tinnitus. If a drug manufacturer were able to create a better alternative, patients and doctors would be grateful, and it would likely be extremely successful.
With their synthesis of aspirin, chemists at Bayer did exactly that.
Aspirin Synthesis Controversy: 1897 to 1949
While the events of the 18th and 19th centuries relate to a verifiable history of aspirin, the next period becomes muddied once again. However, here it does not relate to lost or convoluted records, but prejudice.
We know the following to be absolutely true: Felix Hoffman, a chemist working for Bayer, synthesized pure acetylsalicylic acid (later known as aspirin) from salicylic acid in 1897. His name is on the U.S. patent for aspirin, and his lab notebook details his synthesis of acetylsalicylic acid. Hoffman’s method was relatively simple, reliable, and efficient. Hoffman heated a combination of salicylic acid and acetic anhydride (an acetylating agent) for two hours, which resulted in a clear liquid. Upon cooling, this liquid yielded a mass of acetylsalicylic acid crystals, which he separated out and recrystallized, using dry chloroform to remove any remaining impurities.
The controversy, then, refers both to why Hoffman synthesized aspirin and what happened afterward.
On the one hand, we have the official story from Bayer, which claims that Hoffman created aspirin to help his father, who was suffering from severe rheumatism. Hoffman had treated his father with salicylic acid, but his father had severe side effects, including nausea, gastric irritation, and tinnitus. Hoffman, therefore, set out to produce a purer derivative of salicylic acid that would be as effective. This version of events was first reported in a footnote in a 1934 book on the history of chemical engineering.10
On the other hand, we have a story from Arthur Eichengrün. Eichengrün was a Jewish chemist who also worked at Bayer. In 1949, he wrote a journal article describing why aspirin was synthesized, and what happened after.11
In this article, Eichengrün stated that he was appointed, in 1895, to establish and manage a pharmaceutical laboratory at Bayer. He also claimed that it was he who instructed Hoffman to synthesize acetylsalicylic acid in 1898 and that Hoffman had done so without knowing why. Notably, Eichengrün also claimed that several Bayer chemists made various derivatives of salicylic acid, each of which was tested further.
Drugs developed in Eichengrün’s laboratory were tested at Bayer’s pharmacological laboratory, led by Heinrich Dreser.12 When the salicylic acid derivatives were tested, it was clear that acetylsalicylic acid was the most favorable, producing only minor deleterious effects on a frog heart. However, in a Bayer management meeting to discuss whether acetylsalicylic acid should go forward to clinical trials, Dreser asserted that it was a direct cardiac poison and opposed it progressing to trials. Dreser had the right to veto any drug going to clinical trials, so this is where the story of aspirin could have ended.
However, in this article, Eichengrün stated he couldn’t accept the decision to stop work on acetylsalicylic acid, and, against his contract, continued to conduct tests privately. He tested the drug on himself, then enlisted the help of doctors to test 100 grams of homemade acetylsalicylic acid.13 None of the doctors reported side effects in their patients, and they asked for larger quantities for more detailed testing. From these tests, it was clear that acetylsalicylic acid retained the beneficial clinical effects of salicylic acid, including potent pain relief, but produced fewer side effects.
A report was sent to Bayer detailing the results of these tests, upon which Dreser commented: “the product has no value.” Nonetheless, Bayer decided to conduct more tests, the results of which confirmed that acetylsalicylic acid worked. Eichengrün stated he suggested the name “aspirin.”14
Despite his condemnation of aspirin, Dreser was commissioned to write the paper on Bayer’s synthesis and testing of aspirin, which was published in 1899. His article focused on the pharmacology and animal studies, omitted details of tests in humans, and contains no mention of either Hoffman or Eichengrün. Eichengrün acknowledged this was standard practice at the time: only the company that made the drug was reported, not the inventors.15
While Eichengrün’s article makes it very clear that he and Hoffman should be credited with the invention of aspirin and not Dreser, he waited until 1949 to publish his account. The account with Hoffman as the sole inventor, however, was published in 1934. So why did Eichengrün wait?
In 1999, Walter Sneader published a reappraisal of the discovery of aspirin, in which he looked at Eichengrün’s 1949 article and other relevant documents. In it, Sneader argued that Eichengrün’s ethnicity forced him to remain quiet. Eichengrün was a Jew living in 1930s and 1940s Germany. The Nazis were making life successively more difficult for Jews, even for successful factory owners like Eichengrün. While Eichengrün had hired a gentile associate to avoid the loss of state contracts, his company was forcibly transferred to a non-Jew in 1938. And although 76 and married to an “aryan,” he was interned at Theresienstadt in 1944 for 14 months.
In his 1949 article, Eichengrün recalled that he saw a display for aspirin in the Hall of Honor of the chemical department of the German Museum in Munich in 1941, with the inscription: "Aspirin; Inventors Dreser and Hoffman." Also at the entrance to the museum was a sign prohibiting Non-Aryans from entering. To this, Eichengrün simply stated: “Sapienti sat!” (translated as: “Enough for the wise!”)
Given this context, it’s not difficult to imagine why Eichengrün may have been reluctant to claim credit, even if he were aware of the 1934 book. Aspirin was successful and important: if a Jew were to have claimed credit for its invention, when at every level Jews were being forced out of public life and their accomplishments erased, such a person would have undoubtedly provoked a hostile response.

Additionally, Eichengrün’s article is only the account of one man, written at the very end of his life (he died the same month his article was published), and could be biased, deceptive, or otherwise incorrect. In a press release responding to Sneader’s article, Bayer stated that Eichengrün’s claims cannot be proven. Bayer also claimed that Hoffman was Eichengrün’s equal hierarchically, so Eichengrün couldn’t have ordered Hoffman to create acetylsalicylic acid. The press release also questioned why Eichengrün didn’t object at the time of the patent being awarded solely to Hoffman, and seemed troubled that Eichengrün waited 50 years to claim his role in the development of aspirin.
Against this, we have the knowledge that Nazi censorship, anti-Jewish legislation and sentiment, and propaganda (including in textbooks) could have removed Eichengrün from the history of aspirin. Even this year, we have examples of erasure of accomplishments due to people’s ethnicity, gender, or both.
Ultimately, this controversy remains unresolved. While it is entirely possible that evidence for Eichengrün’s contributions at Bayer were purged due to antisemitism, we cannot be certain.
NSAID Expansion: 1949 to present
While it is seemingly impossible to find figures for aspirin consumption or production over time, it is clear that it took off after Bayer began marketing the drug in 1899. Eichengrün noted that it wasn’t just that aspirin was effective with limited side effects, but also that it came packaged as tablets rather than as a powder in a paper bag. Tablets were a novelty at the end of the 19th century. Additionally, while the trade name “aspirin” was protected from imitation, the process for making it was not. This meant the availability of generic acetylsalicylic acid sold under different names16 soon drove down prices.
Aspirin’s popularity diminished once paracetamol and ibuprofen became available in the late 1950s and early 1960s, as the latter drugs had even fewer gastrointestinal side effects. However, its use rose again after the ISIS-2 trial, published in 1988. This trial tested whether low-dose aspirin and streptokinase, separately or together, were effective at preventing mortality after suspected acute myocardial infarction (heart attack). The results were so unambiguously positive for aspirin (and streptokinase, although aspirin was far cheaper) that aspirin use sharply increased worldwide in acute coronary care. The use of aspirin amongst people in hospitals with acute myocardial infarction, for example, increased in the UK from 10 percent in 1987 to over 90 percent in 1989.
Further studies looked at whether aspirin was effective at preventing cardiovascular disease (including heart attacks and strokes), and led to recommendations promoting low-dose aspirin use. A study using 2017 interview data suggested that 23.4 percent of adults 40 years or older in the U.S. (estimated to be about 29 million people) took daily aspirin to prevent cardiovascular disease. Although there are disputes around who should take aspirin for cardiovascular disease prevention, it nonetheless remains extremely popular.
The origins of other NSAIDs are not nearly as dramatic as aspirin. Starting in the 1960s, multiple NSAIDs were developed and patented: indomethacin (1961), ibuprofen (1962),17 mefenamic acid (1964), naproxen (1967), diclofenac (1978), celecoxib (1993), etoricoxib (1996), among others.18
Despite the numerous brands of NSAIDs, their mechanism of action remained unknown until 1971, when John Vane discovered the mechanism of action of aspirin, and, by extension, other NSAIDs. These molecules, he found, work by blocking cyclooxygenase (COX) enzymes. COX enzymes produce compounds19 that cause pain, inflammation, and fever: if you block them, fewer of those compounds are produced.20 This discovery led to a rapid expansion in the number of available NSAIDs and a Nobel Prize for Vane in 1982.
While future NSAID development is likely to focus on maintaining or improving clinical effectiveness while reducing side effects, there may be novel roles for NSAIDs to fulfil, such as occurred in the 1980s when aspirin was promoted as a treatment and preventative for cardiovascular disease. NSAIDs may likewise play some as yet undetermined role in neurodegenerative diseases, diabetes, and cancer disease therapy.
A Search in the Cabinet
When setting out to write the history of NSAIDs, I thought the project would be reasonably straightforward; people used to drink willow bark tea, and someone clever turned that into aspirin. What I found instead was a labyrinth of oft-repeated myths, partial histories, and conjecture. While piecing the story together, I couldn’t help but feel that scientists could do a better job of preserving their work and methods for posterity.
At its heart, the issue is one of knowing when information is fact, conjecture, or mythology. This is as relevant today as it has ever been, for while the internet has allowed unprecedented access to information, it is as unclear as ever whether that information represents truth. With the rise of artificial intelligence, as well as malicious agents intent on creating and propagating misinformation, determining what is factual will become increasingly more difficult.
Knowing who synthesized aspirin for the first time and why may seem relatively trivial when compared with questions of more immediate relevance and consequence, such as whether facemasks help prevent the spread of COVID-19. But as someone who has attempted to answer both questions, the difficulties in establishing the facts are similar. We need to know who did what, how, and why.
Newton said that scientists stand on the shoulders of giants. However, scientists can only stand above the clouds when there is an unbroken chain of giants beneath them. As soon as one giant is missing, as soon as one link in the unbroken chain of scientific evidence breaks, the whole thing comes tumbling down.
I have one piece of advice for scientists and science communicators: your references matter. They are the evidence that we even have giants supporting us. All scientific claims should be referenced. It isn’t sufficient for you to state that Hippocrates prescribed willow bark tea to treat inflammatory pain. You need to show why you think that is true.
The risk of not providing reputable sources is not just that myths get propagated, it’s that lies get propagated. After all, it likely doesn’t really matter in 2025 whether Hippocrates made people drink 41 cups of willow bark tea a day, or if people write historical fiction where characters rush off to find willow bark to quell a fever. But it does matter whether people believe, for instance, that vaccination is the best way to prevent measles.
I also have one request for people who read scientific literature (of any description): demand better. People may claim to be standing on the shoulders of giants, but are actually shouting from two feet off the ground on a rusty, broken bicycle. Do authors present quality evidence to support their claims, or does it fall apart when you dig a little deeper?
Before researching its origins, I believed that willow bark tea was ancient aspirin. Now, I believe it’s simply willow bark tea. It may behave similarly to aspirin, but not because it is aspirin.
However, by using direct evidence to piece together the rest of the story, I’ve come to trust the following: Reverend Stone found a successful application of powdered willow bark sometime before 1763. He didn’t do this because of any historical precedent, but simply because he thought it might work. Fontana then extracted and named salicin, likely referencing Stone in his report. This led to the use and manufacture of salicylic acid, and then to Arthur Eichengrün, who likely asked Felix Hoffman to synthesize acetylsalicylic acid in 1897.
Ultimately, while I don’t think willow bark tea is ancient aspirin, I do think aspirin can still trace its origins back to the willow bark infusions that Reverend Stone used to treat the people of Chipping Norton over 250 years ago. So fancy that: aspirin was ultimately derived from willow bark after all, maybe just not as long ago as the histories would have us believe.
Sean Harrison is an expert in epidemiology and evidence synthesis. He has spent the last decade answering research questions by finding, analysing, and synthesizing all available evidence to answer those questions. During the COVID-19 pandemic, he worked at the UK Health Security Agency, providing evidence reviews to inform COVID-19 policy. He is currently a research fellow at the University of Exeter and blogs at seanharrison.blog. Find him on BlueSky at @sean-h.bsky.social.
Cite: Harrison, S. “The Uncertain Origins of Aspirin.” Asimov Press (2025). https://doi.org/10.62211/58qw-41hg
We recorded a behind-the-scenes interview with Sean. Watch on YouTube.
Header image by Ella Watkins-Dulaney.
If you have a headache, a sprained ankle, a temperature, or some other minor complaint, there’s a good chance that if you reach for any drug, it’ll either be an NSAID, such as aspirin or ibuprofen, or paracetamol (a different class of drug).
Accurate global statistics are difficult (possibly impossible) to find for the total number of prescribed NSAIDs, but in 2022 in the U.S., ibuprofen was the 33rd most prescribed medication (17.5 million prescriptions), followed by aspirin at 36th (17.0 million), diclofenac at 51st (12.5 million), naproxen at 89th (7.4 million), celecoxib at 93rd (7.0 million), and indomethacin at 256th (1.1 million). Mefenamic acid and indomethacin weren’t in the top 300 most prescribed medications.
There are other, more infrequent references to ancient people using willow to reduce pain, inflammation, or fever found among the Chinese and the indigenous peoples of America and South Africa. Additionally, although it is claimed that an Ancient Assyrian clay tablet (3,500 to 2,000 BC) described the use of willow leaves for pain and inflammation, I haven’t found original evidence for this, nor the current location of the tablet, nor its translation.
The symbol of medicine as a staff with a serpent coiled around it — the staff of Asclepius — may have been derived from this treatment for guinea worm. However, like the rest of the historical details in this article, we can’t be sure. We do know, however, that the “caduceus,” a rod with two snakes coiled around it, often with wings at the top, originally had nothing to do with healing or healthcare. Various healthcare-aligned groups, as well as the U.S. Public Health Service, have adopted it nevertheless, possibly confusing the two symbols. In fairness, the two symbols look similar, and a winged rod with two snakes does look better than a wingless rod with only a single snake.
The Hippocratic oath was named for Hippocrates, although it was unlikely to have been written by Hippocrates, and doesn’t start with “first, do no harm,” as many (including myself, until recently) believe. Whether any of the surviving works attributed to Hippocrates were actually written by him is debatable, given they are anonymous, written at different times by different hands, and have different ideas about the body and healing. Franz Zacharias Ermerins, a Dutch physician and editor, apparently identified at least 19 different authors of works attributed to Hippocrates.
This is, evidently, part of the Doctrine of Signatures. This is the belief that there must be some (divine) sign telling us which plants to use to cure which diseases. Often, it was the appearance of a plant that was the clue: eyebright flowers resemble eyes, so must be able to treat eye issues; liverwort resembles the shape and colour of the liver, so must be able to treat liver issues; lungwort resembles disease lungs, so must be able to treat lung issues. In this case, it was the location of willow trees: “the general maxim, that many natural maladies carry their cures along with them, or that their remedies lie not far from their caufes, was fo very appofite to this particular cafe, that I could not help applying it; and that this might be the intention of Providence here, I muft own had fome little weight with me.”
Cinchona is almost certainly named for the Countess of Chinchón, a Spanish noblewoman married to the Viceroy of Peru, who developed fever and chills (likely malaria) in 1631. Jesuit priests made a remedy that included the bark of the Cinchona tree, which, presumably, previously had a different name. The remedy worked, and the tree was named Cinchona in her honor.
Other plants with six or fewer mentions were buttercup, foxglove, lesser celandine, and pennywort. Notably, foxgloves were the initial source of digoxin, used to treat various heart conditions, including heart failure. The origins of digoxin also involves medical history mythology: William Withering was an 18th century botanist and physician who, in 1785, first wrote about using foxgloves to treat dropsy (edema caused by heart failure, among other things). However, in 1928, the pharmaceutical company manufacturing digoxin invented “Mother Hutton” as part of a marketing campaign. “Mother Hutton” was an old herbalist from Shropshire, who originally discovered that foxglove tea helped dropsy, and who was paid by Withering for that information. Many people now believe the myth that “Mother Hutton” first used foxglove tea for dropsy, despite her being entirely fictional.
Charles Gerhardt was credited with the first synthesis of aspirin in 1852. He reportedly reacted sodium salicylate with acetyl chloride, creating acetylsalicylic acid. However, the final compound was unstable and impure, and the process to make it inconsistent and cumbersome, so Gerhardt reportedly did not investigate further. I was unable to verify this when translating the original evidence: it is both technical and in German, so I’m leaving this as “reportedly.” It’s one of the challenges of working with 19th century foreign-language technical journal articles …
I’ve been unable to access a copy.
I went to some length to find a copy of this article. It didn’t exist online, but I found a physical copy for sale on eBay in Poland. Unfortunately, the seller wouldn’t initially ship to me in the UK, so I used Google Translate to ask them (in German) to allow shipping to the UK, which they very kindly did. Subsequently, DHL managed to lose the parcel, which is a shame as it was one of the few extant copies of this article in existence. However, the British Library is, thankfully, magnificent: they had a copy, and someone very generously managed to dig it out of the archives and photograph it for me. I transcribed the German text, translated it with Google translate, and put both the original German transcription and the English translation on the Internet Archive for posterity. Weeks later, DHL found the article and delivered it, so I scanned the original and uploaded it to the Internet Archive.
Eichengrün did not go into specifics of these tests in his article. Dreser’s introductory paper on aspirin mentions a number of different tests, but it is unclear when they were conducted, and by whom. Nonetheless, Dreser mentions tests using acids and alkalis, taking aspirin himself and then testing his urine (concluding the aspirin had turned into salicylic acid), and tests in rabbits (including body temperature), frogs (including blood vessel constriction, isolated heart function, and lethal dosage), and fish (including lethal dosage). Other tests may have been conducted that were not reported in this paper.
This, quite obviously, could have gone horrendously wrong: thankfully, clinical testing of new drugs has far stronger safeguards these days.
“Aspirin” is derived from “a-” for acetyl, “-spir-” from “Spirsaüre” (spiric acid, an older name for salicylic acid, derived from the Latin for meadowsweet: Spiraea ulmaria), and “-in”, a common chemical suffix.
Eichengrün also stated that they couldn’t patent aspirin in Germany as only the preparation process, not the end result, could be patented. The process for making aspirin, however, also couldn’t be patented because an earlier publication described how acetyl chloride had been mixed with salicylic acid under pressure in a gun barrel. Although the end result was not described, this was evidently sufficient grounds to not award a patent for the process of making aspirin. As a result, and because Bayer would only financially compensate Eichengrün and Hoffman if a patent were granted for a new drug, neither benefited financially from creating aspirin.
For example, salicyl acetate and acidum acetylo-salicylicum: the latter I imagine being a challenge for anyone to pronounce quickly and easily upon first reading it.
The development of ibuprofen shares striking similarities to the development of aspirin. Ibuprofen was developed while searching for a derivative of aspirin that would be suitable for long-term use for rheumatoid arthritis with fewer side effects, much as aspirin was developed as an alternative to salicylic acid. The lead scientist, Dr Stewart Adams, was also happy to test the compounds on himself first (after toxicity tests), and was reportedly “excited to be the first person to take a dose of ibuprofen”, much as Eichengrün first tested aspirin on himself.
I’d be lying if I said I knew all those drug names before writing this article.
Thromboxanes, which play a role in platelet adhesion; prostaglandins, which cause vasodilation (expansion of blood vessels), increase body temperature by interacting with the hypothalamus, and reduce pain; and prostacyclins, which inhibit platelet activation and cause vasodilation.
There are two cyclooxygenase (COX) enzymes, which are both affected to a greater or lesser extent by NSAIDs. The first of which (COX-1) is found all over the body (in particular the gastrointestinal lining and kidneys), and the second (COX-2) is only around during an inflammatory response. As they affect these two enzymes differently, NSAIDs have different levels of effectiveness and side effect profiles.










Wonderful eesay ,however it would be more insightful if u had included ayurvedic indian origins and Chinese medicine origins of the this drug the article would b more insightful and whole some.keep up the good work...
This is one of my favourite genre of essays: someone goes really, really far to check if a common claim is true. It takes so much work!