To Be Born in a Bag
Despite recent progress, artificial wombs still face technical challenges. De-extinction efforts, of all things, are driving it forward. Just how much is anyone’s guess.
Tom Ireland writes about technological advances — and roadblocks — in artificial wombs for Issue 04 of Asimov Press.
The womb is a remarkable organ — a muscular, pear-shaped chamber that supports the transformation of a tiny cluster of dividing cells into an entirely new person. All humans begin their lives in this sturdy chamber.
At least for now.
Several research groups are busily developing artificial wombs to replicate the basic life-support functions of the uterine environment for extremely premature infants — that is, babies born at or before 28 weeks of the typical 37-42 week pregnancy.1
At least one group is working on something altogether more radical: a system to grow a fetus from embryo to birth entirely outside the womb, a concept known as ectogenesis. And while currently being developed under the guise of helping to rescue extinct or critically endangered species, such technology could have profound implications for the future of human reproduction.
In 2018, researchers at the Children’s Hospital of Philadelphia managed to support the growth of fetal lambs for up to 4 weeks outside the womb,2 using an artificial womb to replicate the gaseous exchange function of the placenta, oxygenating the fetus’ blood and removing carbon dioxide. Results such as these have been so encouraging that in September of last year, the FDA began to review the idea of moving to human trials.
Despite this enthusiasm, however, a number of technological challenges remain before scientists can test such wombs on human babies. Premature infants differ from animal fetuses in both physiology and how willing we are to subject them to risk. Indeed, the stakes in the first real-world use of artificial wombs may be higher than in almost any clinical trial imaginable.
As for the more ambitious idea of growing an animal from embryo to birth outside the womb, challenges remain even to get to animal trials. Scientists must synthetically replicate the process that encourages an embryo to grow a physical connection to its mother, a developmental phase that is still poorly understood. Next, they need to devise an artificial interface that replicates the complexities of the endometrium, the lining of the uterus that connects to the placenta. From there, the system must be able to change scale as the embryo grows from the size of a grain of rice to a wriggling full-term infant. And finally, such an environment must be able to mimic the complex mix of antibodies, hormones, growth factors and other important molecules transmitted to the developing fetus in utero.
Despite these hurdles, it might not be very long before the first human baby is birthed from a bag. At least five research groups are actively working on artificial womb technology to support babies born before week 24 of the 40-week full term — that crucial point at which the chances of survival fall dramatically (from 70 to 30 percent, according to one study) and long term health complications are almost certain for those preemies who survive.
These include groups in Michigan, Philadelphia, and Toronto, the Perinatal Life Support project in Europe, and the EVE collaboration between groups in Australia and Japan. A research team led by Alan Flake, a pediatric surgeon at the Children’s Hospital of Philadelphia, has entered one-on-one discussions with the FDA about testing their artificial womb, known as EXTEND (the Extra-uterine Environment for Neonatal Development), on a human baby.
The question, however, is not limited to whether these devices are ready to be tested on humans, but if we are ready for the technology.
Cusp of Viability
Hospitals need new ways to care for extremely premature infants. According to the World Health Organization, complications related to preterm birth caused about 900,000 deaths in 2019, and even in the United States, where standards of care lead the rest of the world, about one-third of infants born at 24 weeks will die.
Of those born at 22 weeks — considered the cusp of fetal viability — just 30 percent survive, almost all with life-long health complications. The current gold standard of care for these extremely premature babies employs a ventilated face mask or a breathing tube inserted into their windpipe to pump oxygen into their lungs. The baby is warmed under a heat lamp, with fluids and nutrition provided through an intravenous line.
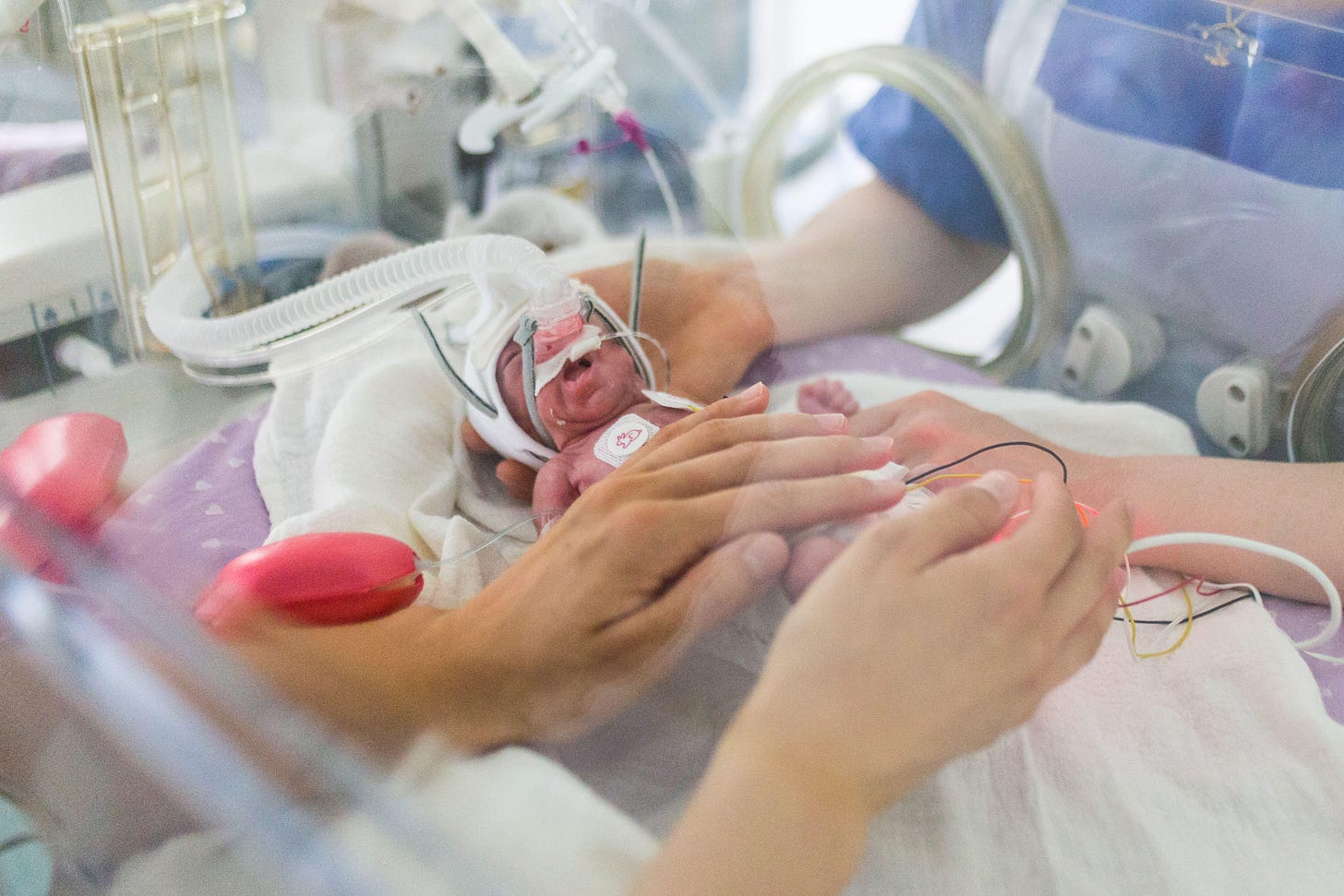
At this point, however, infant lungs are not ready to handle gas exchange, even with special low-pressure ventilators. Despite the use of steroids to reduce the impact of mechanical ventilation, irreparable damage to babies’ delicate and underdeveloped lung tissue often results, causing long-term breathing conditions.
Artificial wombs offer a different approach — aiming to replicate the role of the placenta, providing oxygen and removing carbon dioxide from the blood, and allowing the lungs to remain submerged as they would in the womb. The devices vary in complexity, but all center around what is known to medics as “extracorporeal membrane oxygenation,” or ECMO — colloquially known as a heart and lung machine.
In this form of life-support, often used during surgery or in intensive care, blood is taken out through a cannula and pumped through a type of artificial lung called a membrane oxygenator. This adds oxygen to the blood and removes carbon dioxide. The blood is then warmed to body temperature before re-entering the body through another tube.
In artificial wombs, an ECMO circuit is miniaturized in proportion to the blood vessels and blood flow of a fetal animal or very premature baby. Most approaches involve placing the fetus in a fluid-filled bag, sac, or pod, mimicking the uterine environment by keeping the fetus submerged in an amniotic fluid substitute. Images published as part of the EXTEND project show a young lamb, eyes closed, seemingly shrink-wrapped within what looks like a zip-lock bag of fluid, with tubes snaking in and out.
Across the Atlantic, scientists and engineers at the Maxima Medical Centre in the Netherlands have developed eerily life-like fetal dolls to test their artificial womb prototypes — blood-red plastic pouches that expand into suspended, balloon-like pods as the baby grows.
A simpler approach forgoes the fluid-filled bag altogether, with an animal fetus hooked up directly to a small ECMO circuit in a conventional neonatal incubator, a small boxy chamber for thermoregulation. This technique requires that only the lungs of a fetus be filled with special growth-stimulating fluid. Less of a departure from existing preterm birth life-support, the bag-free approach might be more straightforward for healthcare systems to test and adopt.

Although the “baby in a bag”-style systems make access to the fetus more difficult for medical providers than the bagless approach, being submerged in fluid may have advantages in terms of reducing stress, cushioning the baby and its developing organs, and protecting its delicate skin — a common source of infection and injury in premature infants. This is largely because preemies still lack the “cheesy varnish” known as vernix caseosa (which forms primarily during the last trimester of pregnancy) that hydrates the skin, maintains pH, and contains various antimicrobial compounds. Their skin is also thinner, which makes them extremely vulnerable to the physical, medical, and microbiological incursions of the neonatal ward.
Ultimately, whichever system is used, researchers must account for gas exchange, growth, and nutrition — challenges that are by no means new.
Incubating an Idea
Despite sounding futuristic, efforts to make artificial wombs stretch back to the 1950s. Soon after the first heart-lung machine was used to oxygenate a patient’s blood during heart surgery in 1953, researchers began to study other ways they might be able to use “extracorporeal circulation.” It did not take long for a worthy use case to present itself — helping premature babies. Fetuses do, after all, enjoy a kind of extracorporeal circulation powered by their mother’s heart and lungs.
The first patent for a complete artificial womb was filed in 1954. The design included a tank filled with amniotic fluid, a synthetic umbilical cord, blood pumps, an artificial kidney, and a water heater. And the first test of that artificial womb took place in 1958, in an experimental procedure that would make modern ethics boards wince.

A team in Stockholm, led by obstetrics professor Bjorn Westin, connected seven “pre-viable human fetuses,” obtained from aborted pregnancies, to a simple circuit that pulled blood from a vein in the umbilical cord, oxygenated it, and returned it into the body. The fetuses were also submerged in an isotonic glucose solution to mimic amniotic fluid in a closed system resembling a stove-top pressure cooker. These fetuses survived for between five and 12 hours.
Further studies of similar devices continued in the 1960s, with animal rather than human fetuses. This included the first delivery of a live animal, a lamb transferred from its mother’s womb and born, seemingly healthy, “after 40 minutes in the artificial placenta.” These early studies typically used a simple blood reservoir elevated above the fetus and an oxygenator to suffuse oxygen into the blood before it returned to the body. By 1969, scientists were able to keep a premature lamb alive for over 50 hours.
While historically, artificial wombs focused on keeping premature infants alive long enough to continue developing, today, researchers are trying to make artificial wombs that replicate the human womb completely.
With the number of significantly premature infants born in the U.S. rising (rates of preterm birth have risen by 12 percent from 2014 to 2022, a trend seen in many other countries) and poor prognoses expected for those born before 24 weeks, several research groups are attempting to hasten progress on artificial wombs. But even buoyed by refinements in ECMO machinery and prenatal care, these researchers face significant hurdles.
One of the largest of these is miniaturization. The animal model commonly used to study artificial wombs is the fetal lamb, often placed in the devices at around the 115th day of its typical 145-day gestation. The lamb’s level of lung development here is strikingly similar to that of a 22 or 23-week-old baby. The problem is, the lamb is huge relative to a baby — weighing as much as three and half kilograms (8 lb) while many preemies may weigh less than half a kilo (just over 1 lb).
For a human infant, the whole system needs to be smaller — from the tiny cannulas used to connect minuscule blood vessels to the oxygenators that work with extremely delicate blood flows and pressures. Get it wrong, and the system will create dangerous fluctuations in blood pressure. The cannulas could trigger what is known as umbilical spasm — the tendency for the arteries of the baby’s vascular lifeline to close tightly when injured or exposed to air. The miniaturized system must also be able to adapt to the rapid growth that will occur in the coming weeks when the baby will almost double in size.
“Many people would say, ‘miniaturizing it, no big deal’,” says George Mychaliska, a professor of pediatric surgery currently developing an artificial womb procedure at the University of Michigan. “It can be done, and we're making progress, but it's not easy.”
The risk of umbilical spasm is so serious that it has led some physicians to elect not to connect their system to the umbilical cord, but instead to blood vessels used in standard adult ECMO, such as the jugular vein. However, this method requires the system to have an external pump, whereas, in the umbilical model, the fetal heart provides this function.
Another major issue in human babies is blood clotting. Blood tends to coagulate when it comes into contact with foreign materials, and for life-support systems like ECMO to work in adults, the blood is typically thinned using drugs such as heparin.
However, thinning the blood is risky for very premature babies as they are already at heightened risk of bleeding in the brain. In the delicate brains of babies younger than 28 weeks, a highly vascularized area known as the germinal matrix is underdeveloped and particularly susceptible to hemorrhage. Because this type of hemorrhage occurs in up to 20 percent of very low-birth-weight preterm babies, pediatricians rarely provide ECMO to preemies younger than 29 or 30 weeks.
Solutions will likely necessitate the development of ECMO systems that do not require thinned blood to function properly. One promising option may come in the form of polymer coatings that release nitric oxide — a natural anticoagulant — as the blood passes through. Such coatings have been in development for years, but mostly to complement blood thinning treatments during ECMO, rather than replace them. However, in a recent study using sheep, a team at the University of Michigan Medical School found that a nitric-oxide-rich ECMO circuit allowed them to operate an artificial womb without blood thinners.
Finally, transferring the baby to the artificial womb poses several challenges. Chief among these is how to keep the baby in a fetal state, avoiding the physiological changes that are triggered when a baby is born and takes its first breath. Ideally, the transfer should be done in a way that shields the baby from temperature change, air, light and loud noise, and any other trauma.
In the EXTEND system, considered one of the most sophisticated approaches to artificial wombs, physicians perform a cesarean on the maternal sheep under general anesthetic, and give the fetus opioid painkilling injections. Surgeons quickly connect the fetus to the ECMO circuit with the umbilical cord still attached, and the cord is severed from the mother only after a successful blood flow is established. Due to its complexity, this surgery is not something that can be done in an emergency or following an unexpectedly preterm birth. Caesareans are also riskier in these earlier stages of pregnancy when the wall of the uterus is thicker.
Scientists at the Eindhoven University of Technology have started to model one transfer technique that involves babies being delivered through the birth canal straight into a fluid-filled “transfer pouch” and another that pulls the baby straight into the pouch from the uterus via a cesarean incision. Their work would, theoretically, mean the procedure could be used in a wider variety of preterm birth scenarios, but the connection of the umbilical vessels would then have to be performed while the baby is in the fluid-filled pouch, complicating an already delicate surgery.
Whichever model works best, the fact remains that transferring a baby into an enclosed, liquid-filled system while also providing medical care represents an entirely new paradigm for medical personnel working in this field, potentially adding risk for both mother and baby. By contrast, a bagless version that avoids the umbilical cord would offer a simpler transfer that could be attempted after efforts at more conventional types of care have failed.
Engineering Challenges
Of all the groups working on artificial wombs, the team behind the EXTEND device, at the Children’s Hospital of Philadelphia, appears closest to solving these issues. As well as their one-on-one talks with the FDA, the group is now commercializing its technology through a well-funded start-up, Vitara Biomedical. However, Dr. Flake, who leads the work, has refused to talk publicly about how close his team is to human trials.3 Meanwhile, other researchers have advised the FDA that research results do not yet warrant moving to this stage.
Matt Kemp, the lead investigator of an artificial womb project at Australia's Women and Infants Research Foundation, wrote in his evidence for last year’s FDA review that no data yet suggests the new approach can support an extremely preterm human fetus “with better results than standard neonatal care available today.”
In particular, Kemp has concerns surrounding the lack of studies involving animals of comparable birth weight to the infants that would be the most likely candidates for this type of treatment. He points to the fact that “repeated heart failure and death” occur when incubating extremely premature lambs (with an average weight of under 650g) in artificial wombs and to his own research that found evidence of serious growth failure when using similarly-sized lambs.
In the Netherlands, a collaborative project known as Perinatal Life Support is avoiding animal studies (at least for now) by conducting tests using computational modeling and silicon “babies” fitted with sensors and moving limbs. It remains to be seen how data generated this way might be regarded by regulators.
Kemp also notes that none of the groups working on artificial wombs for premature babies are even attempting to replicate the complex immunological and hormonal signals that the developing fetus receives from its mother in the womb. The reason, disappointing as it seems, is that these signals are still too poorly understood — a recognition prompting some researchers to prefer the term “artificial placenta” in acknowledgment that their devices are really only providing mechanical life-support functions rather than replicating the complex chemical environment of a natural womb.
If these difficulties weren’t formidable enough, there is also the troubling question of which infants will actually be candidates for this new form of care. Preemies 24 weeks or older are more likely to survive with current care. Testing an experimental approach on babies that may have good outcomes without it seems unethical. But the youngest, most premature infants are often the sickest, likely to be suffering from complications of infection or inflammation, reducing the odds of their surviving the physiological demands of being connected to an ECMO system.
Complicating this further is the fact that outcomes differ significantly across the U.S., with some leading hospitals able to keep twice as many 22-week-old babies alive as the national average, and occasionally able to keep babies born as early as 21 weeks alive. This makes it difficult to make general guidelines about the point at which physicians should try an artificial womb over conventional care.
Despite the many challenges, Kemp’s conclusion is hopeful: “If the above factors can be successfully addressed, as they have been with no small degree of success in the current gold-standard ventilation approach, there would be good justification for human trials of [artificial womb] technology. We hope, with further effort, that such a scenario could occur within the next 5–10 years.”
Others have much more ambitious plans for the next five years.
The disruptive biotech company Colossal Biosciences is best known for its headline-grabbing “de-extinction” project, with the stated goal of creating a living woolly mammoth by 2027.
By combining mammoth DNA recovered from ancient permafrost with stem cells of the species’ closest living ancestor, the Asian elephant, the company aims to make hybrid embryos. These embryos must then be nurtured and able to grow to term, ideally inside of an Asian elephant. But Asian elephants are endangered, a status that greatly constrains their use as experimental surrogate mothers. To make these chimeric creatures, then, Colossal must also create its own womb. Or, as they call it, a “complete ectogenesis system.”

Mention the term “ectogenesis” to the researchers working on artificial wombs for premature babies, and they often roll their eyes. They will tell you that the idea is absurd, technically naïve, or even outright impossible; nobody is trying to grow babies in a lab without a mother. But that is exactly what Colossal plans to do — in what is almost a side-project of their de-extinction venture, scientists at Colossal are developing artificial wombs to grow living creatures from embryo to birth.
The development of such a system could help conservationists replace animals in the numbers required to restore lost biodiversity in a meaningful way, says Colossal’s co-founder Ben Lamm. “I truly believe that our success with artificial wombs is the biggest game changer to conservation that will ever exist,” he says. “If you could grow 50 genetically diverse northern white rhinos in a lab, safely, and then work with rewilding partners to put them back in the wild … That would change conservation forever.”
It could also change humanity forever. If Colossal’s artificial womb technology were to work, interest in applying it to humans would follow, particularly in the context of expanding the options available to prospective parents who cannot have biological children. Combining artificial wombs with other fast-moving reproductive technologies such as cloning or artificial gametogenesis could mean both the creation and birth of humans outside the womb. And while there are currently regulatory barriers to many such reproductive technologies, as human embryos cannot be cultured beyond 14 days in most countries according to international guidelines (and national laws, in the case of the UK) — there have been calls to relax this limit.
The amount of work required to recreate the entire function of the maternal womb from the attachment of an early embryo to the birth of the developed infant is dizzying — a goal one would expect to take decades of international scientific collaboration. But Colossal’s founders, Harvard geneticist George Church and software engineer Lamm, have a Musk-like zeal, claiming that what others say is impossible can not only be done but in double-quick time.
Understanding the company’s actual progress toward this goal, however, is difficult. Claims that the “resurrection of the mammoth” is close have been made repeatedly, the first as far back as 2011,4 and the scientists involved tend to publish more press releases than peer-reviewed papers. But Lamm tells me that their scientists are currently working on mouse and marsupial models. The former are fast-growing and well-understood model organisms, and the latter have relatively simple, non-placental wombs, which the fetus leaves early to be supported in a pouch. They plan to crack artificial wombs in these relatively simple systems before advancing to mammals such as lambs or pigs. Once achieved, they will move on to the almighty mammoth calf, which is likely to weigh at least as much as an Asian elephant calf at full term — over 100kg (220 lb).
Keeping an embryo alive ex utero to the point that it develops into a fetus would be unprecedented, and there is little pre-existing scientific or technological progress for Colossal to build upon. A group at the Weizmann Institute of Science in Israel claimed in 2021 that they had developed an ectogenesis device that could support five-day-old mouse embryos for six days — around halfway to their full gestation period of ~20 days. But the device involved rotating vials of fluid, with nutrients and gases simply diffusing into the developing embryos. The animals had no chance of surviving once they were large enough to require a vascular blood supply. Primate embryos have been kept alive for 25 days, reaching a slightly earlier developmental stage than the aforementioned mouse embryos. Still, again — there was no possibility of these embryos developing into fetuses with the techniques used.
To move from the embryonic to fetal, Colossal will need to replicate the complex interface between the placenta and womb lining, where nutrients, gases, and waste are exchanged between the mother and baby. This is where Lamm starts to sound vague, speaking of the need to develop “something like a top dialysis machine, times a hundred.” The most sophisticated dialysis machines use a complex of pores and membranes to remove waste products from patients’ blood while also balancing minerals and electrolytes, but they don’t oxygenate the blood or provide nutrition. Presumably, Lamm aims to fill in these gaps. In 2022, Colossal co-founder Church told Wired magazine that the artificial wombs will be lined with tissue derived from stem cells, but suggested they had not yet actually been able to make those cells.
To support a mammoth, Colossal’s system would also need to be scalable from an embryo the size of a grain of rice to an elephant calf, weighing roughly the same as a large man. The group’s scientists would also need to understand and synthetically replicate the complex signaling environment that helps a developing blastocyst attach, mature into an embryo, and grow a placenta. Additionally, they would need to produce the complex chemical signaling that follows at all stages of fetal development in each species they are working with. This does not faze Lamm.
“These aren’t science gates, these are engineering challenges,” he says confidently — though he admits he is not a biologist and jokes that his scientists have asked him to stop putting timelines on the work.
A New IVF
It remains to be seen if Colossal can hit its absurdly ambitious schedule, or if the FDA’s Pediatric Advisory Committee feels confident enough to start planning the first trials of a womb-like device to support preterm infants. However, it does seem likely that, soon, the point at which a human fetus can survive outside the womb will change. This would not only have implications for medicine but for politics and life beyond the clinic.
It could, for example, challenge the definition of “viability” that has been central to the legal frameworks for abortion. And, as with any cutting-edge form of medical care, concerns will emerge that artificial wombs will only be available to the wealthy.
If, however, issues of cost and accessibility are surmounted, artificial womb technology could potentially expand the options available to those who are unable to carry children. While fertility treatments centered on egg viability, fertilization, and implantation are mainstream, there are currently few options other than surrogacy for those unable to have children because of endometriosis, cancer, or other diseases and disorders that affect the womb or cervix. The technology could also provide an alternative to elective abortions for women who face a high risk of fetal damage during certain stages of their pregnancy.
The expansion of reproductive choice could be utopian, extending fertility windows and allowing more people to become parents, but it will likely be much more complicated. Some ethicists argue that womb-replacement technology would change the role of physicians from “rescuers of life” to “creators of it,” effectively making them pseudo-parents of the developing embryo and fetus.
Indeed, many questions arise as this technology continues at pace. What might it mean for someone to know they were conceived and born artificially? Could artificial wombs help liberate women from the burdens of their physiological role in reproduction? Or might a society where children can be born this way value women less? And then there’s the issue of how such technology might be used nefariously, such as breeding genetically modified people, en masse, for use as soldiers or slaves.
It’s worth remembering that there was once a time when the idea of a baby conceived in a test tube was the stuff of science fiction. When it became a reality, in the late 1970s, it prompted a moral panic that “all hell would break loose” and assembly lines of fetuses would be grown in test tubes or discarded. None of those concerns have transpired, and IVF is now a widely accepted and reliable technology that has brought joy to millions.
The same could be true of artificial womb technology in the future. While the technical challenges standing in the way of artificial womb technology remain significant, many of them look set to be resolved in a matter of years. As research scientists are able to keep embryos alive longer and longer, and artificial wombs help keep premature babies alive earlier and earlier, the window in which we need a natural womb to incubate a baby will get ever smaller.
Join these two timeframes — with the help of an ambitious and disruptive start-up perhaps — and babies conceived and born entirely outside of a maternal womb may be with us sooner than we think.
Tom Ireland is an award-winning science journalist. He is editor of The Biologist, a magazine published by the Royal Society of Biology, and author of The Good Virus: The Amazing Story and Forgotten Promise of the Phage.
Video credits: The video of a premature lamb in an extra-uterine system is from Partridge E.A. et al. in Nature Communications (2017). The video of a non-mechanical artificial womb is courtesy of the University of Michigan.
Cite: Tom Ireland. “To Be Born in a Bag.” Asimov Press (2024). DOI: https://doi.org/10.62211/72hr-98tu
Footnotes
When referring to premature babies, I use the terms “baby” and “infant” as well as “premature” and “preterm” interchangeably, simply to avoid repetition. The term “fetus” is used for a baby that is either still in the womb or born way before the gestation point where they might survive, which is around 21 weeks.
In animal experiments, preterm calves taken from their mother’s wombs and placed in artificial wombs are generally described as “fetal” or “fetuses.” However, a preterm human fetus placed in an artificial womb is likely to be considered “born,” even if the aim is to keep it in a fetal state. It is therefore referred to as a “premature baby.”
Flake declined to talk to Asimov Press for this piece, and MIT Technology Review reports that he declined to answer a question on his progress during the public portion of the FDA meeting in September.
In 2011 a group of Russian and Japanese scientists claimed a woolly mammoth would be born within five years. In 2017 it was reported that Professor George Church was “on the brink” of resurrecting the woolly mammoth; in 2021, Church co-founded Colossal and announced the first mammoth would be born by 2027. Earlier this year, the lead scientist from Revive and Restore, the non-profit which worked on the woolly mammoth project before Colossal took over, cast doubt on that latest timeline.

This is a monumentally bad idea. The end goal is the creation of a human, and that has massive long-term social and ethical implications
- The idea that the contents of the umbilical cord is the sole factor in viability is pathetic and comical. It may be adequate for creating a biologically intact organism, but the “host” mother provides much more than liquid.
- Many adult pathologies stem from attachment failures. Building that in is insanity.
- The Supreme Court has supported property rights to genomes. One could easily harvest an egg and “corporatize” it through the process of gestation in a bag, resulting in corporate ownership of the “end product”
- Read Huxley
- The antinatal argument is not illogical - that life is largely miserable, that the fetus did not consent to birth, and therefore conception is unethical. I don’t subscribe to it, but I would if gestation became divorced from any decision by a parent. If a medical group could take an egg and create a person without the nominal participation of another consenting human, that would be 100% unethical.
- The last control we have in society is control over reproduction. We can deprive the capital system of labor. If babies can be created on demand by capital, it will result in a labor hellscape.
Again. Bad idea
I wonder what sort of implications the existence of artificial wombs would have on the abortion debate. A lot of the standards for regulating abortion have to do with viability, with 24 weeks being the arbitrary cutoff set in the early 70’s and more recent medical technology pushing the actual viability date a few weeks earlier.
Imagine if there were artificial wombs that were functional all the way down to conception, or close to it. In the same way almost no one would support abortions a day before birth, I wonder what people’s opinions would be if it were viable to keep a fetus alive at basically any point.
Maybe it would create far more division, as pro-choice people dig in, or maybe it would solve the issue, as abortion is replaced by transplantation to an artificial womb.