A Most Important Mustard
On the origins of Arabidopsis thaliana, the premier model for plant biology.
This essay will appear in our forthcoming book, “Making the Modern Laboratory,” which tells the story of how the various tools, materials, and methods of the molecular biology lab arrived there and how they might evolve in the future.
The Harz Mountains, whose low ridges sprawl across northern Germany, have been said to be teeming with witches. The poet Goethe wrote in Faust: “Da drängen sich Hexen zu tausend zuhauf” (“Witches throng together by the thousand”). But while such a coven has never actually been found in the Harz, something even more storied has — Arabidopsis thaliana, the premier model for plant biology.
Arabidopsis thaliana was first described by Johannes Thal, a German doctor and botanist born in 1542, who stumbled across it while on an alpine walk.1 From a young age, Thal had been transfixed by the nature of the Harz, collecting and cataloging grasses, herbs, and various resinous plants that might prove medically useful.2 No species was too unremarkable for Thal, fortunate given that Arabidopsis thaliana resembles one of those spindly flowers that sprout between cracks on sidewalks or in the window wells of abandoned cars.

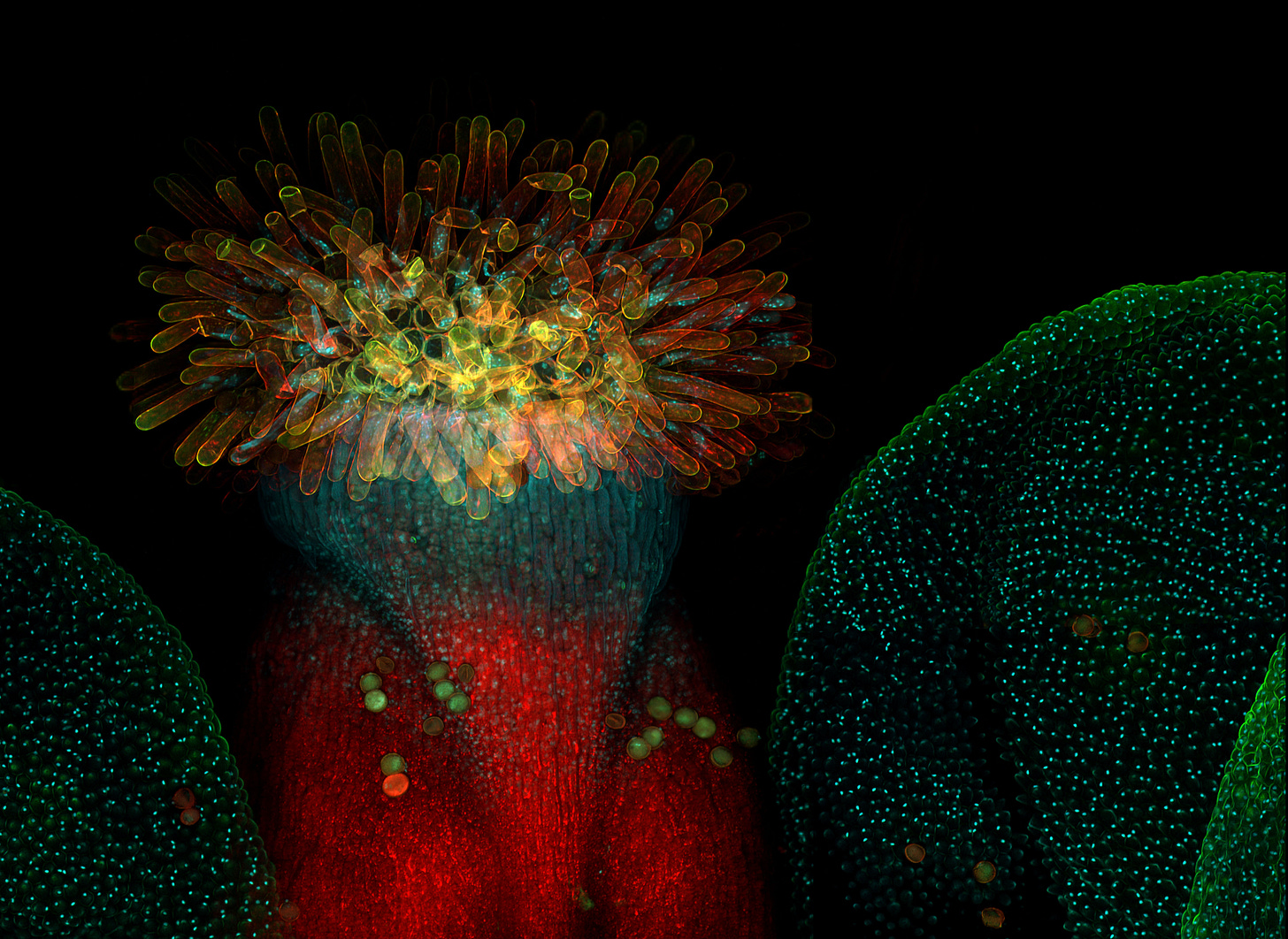
While it may look like a weed, Arabidopsis belongs to the Brassicaceae, or mustard family. Native throughout Africa and Eurasia, it typically grows in rocky, sandy, and chalky soils. Sometimes called “thale cress” or “mouse-ear” cress, Arabidopsis does indeed have flowers whose pale petals resemble the soft, rounded tip of a mouse’s ear. Atop a 20-30 centimeter leaf-studded shoot grow several pale flowers, each with four petals arranged in a whorl.
Although Thal was the first person to pay any mind to this unprepossessing plant, another German botanist, Friedrich Laibach, took the first steps to bring Arabidopsis from the herbarium into the modern research laboratory. As part of his work as a Ph.D. candidate in Bonn in the early 1900s, Laibach spent his time analyzing the number of chromosomes in the various plants that he had collected around the city and his hometown of Limburg. He did this by staining the plant tissue with a special dye that binds to chromatin, the mixture of DNA and proteins that form chromosomes. By examining cells during meiosis — when chromosomes are most condensed and distinct — he could directly count the number of chromosomes possessed by each species. When Laibach turned his attention to Arabidopsis, he found that the plant carried only five pairs, one of the smallest numbers known at the time.
This genetic simplicity captivated Laibach, who began to collect seeds from every Arabidopsis type he could get his hands on, imploring colleagues to look out for new species while traveling. Between 1930 and 1950, he collected seeds from over 150 different ecotypes (later called “accessions”), which he maintained at the Botanical Institute at Goethe University in Frankfurt.
In 1943, Laibach published a paper in which he made a case for the use of the plant as a genetic model. A. thaliana, he asserted, was not only easily cultivated year-round but also self-fertilizing, meaning pure lines could be easily maintained. It was also able to be cross-pollinated by hand, which made it well-suited to experimental breeding. Finally, its small genome made it ideal for cytological and genetic studies, since its hereditary factors could be more easily traced.
The plant’s reception, however, was lukewarm. In the 1940s, plant biology was dominated by economically important crops like maize, wheat, and tobacco, which already had established research communities and clear agricultural relevance. Few agreed with Laibach’s evaluation of the small weedy plant.
One exception was Hungarian plant biologist György Rédei, who read Laibach’s article in 1955 and recognized Arabidopsis’s potential for genetic studies. He obtained four natural accessions (Graz, Limburg, Estland, and Landsberg) from Laibach and brought them to the University of Missouri in Columbia when he left Europe. For the next 20 years, Rédei remained the only researcher working on Arabidopsis in the United States.
While working in a lab he inherited from Nobel Prize-winning cytogeneticist Barbara McClintock, Rédei used radiation to create Arabidopsis mutants with stunted growth. He hoped that by studying the inheritance patterns of this trait, he could map the genes responsible for developmental processes such as stem elongation, hormone regulation, or cell division.
As he worked with these mutants, Rédei discovered that the Landsberg population he had received from Laibach contained genetic variation accumulated in the wild. To establish stable reference lines for future work, Rédei selected two different plants from this heterogeneous population. The first was from the collection of Landsberg seeds that he had irradiated. From these, Rédei selected a line dubbed “Landsberg erecta” after the upright inflorescences (arrangement of the components in the flower head) of the originating mutant. A few years later, he established another line, Columbia (Col-0), which would become the other major reference strain and the one still used to this day.
Enthusiasm for Arabidopsis research remained tepid in the United States during the 60s and sparked only slightly more attention in Germany. In 1964, the eminent plant scientist Gerhard Röbbelen published the first Arabidopsis Information Service (AIS) newsletter to connect the small, but fervent Arabidopsis community.3 The newsletter also acted as a seedstock, including Laibach’s original accessions from decades earlier. A year later, in 1965, Röbbelen organized the first international Arabidopsis conference in Göttingen — attracting just 25 participants.
This modest attendance wasn’t so much a marker of exclusivity as a reflection of the ambivalence still surrounding the value of this plant model. During this period, plant biologists were not only preoccupied with agriculturally important plants, but also believed that tissue culture — the ability to grow and manipulate plant cells in laboratory dishes — would be essential for the future of plant biology. Plants like petunia and tobacco were favored because researchers could readily culture their cells, manipulate them, and then regenerate whole plants from those cultured cells. Attempts to do this with Arabidopsis, however, were frustrating; members of the Brassicaceae family do not grow well in dishes, often developing with deformed or fused leaves.
In the late 1960s and 70s, the world of plant research didn’t have any interest to spare for Arabidopsis. Researchers were busy puzzling over the bacterial pathogen Agrobacterium tumefaciens, which caused tumor-like growths on many plants. In 1969, Australian plant pathologist Allen Kerr recognized that bacteria could pass the tumor-causing trait to each other, writing that “this seems to be the first unequivocal evidence for transfer of virulence from a plant pathogenic to a saprophytic species of bacteria … ”
Shortly after, studies by groups including Jeff Schell and Marc van Montagu in Belgium, and Mary-Dell Chilton in Eugene Nester’s laboratory in the United States, demonstrated the relationship between crown gall disease and the tumor-inducing plasmid (Ti plasmid). Taken together, these discoveries pointed toward the radical hypothesis that A. tumefaciens transfers its own genetic material to plants, and that it might even be possible to exploit this natural process to transfer genes into plants.
A paper on the first transgenic Arabidopsis plant was published in May of 1986, and another followed just five months after from a team at Monsanto, quickly sparking excitement over its genetic makeup. Its authors noted that “The low incidence of repetitive DNA and small genome of A. thaliana suggest several potential uses for the more than 100 independently derived transgenic plants obtained with this procedure,” and concluded that “Demonstrations of the feasibility of these approaches to the identification, isolation, and analysis of specific plant genes will guarantee the position of A. thaliana as ‘the Escherichia coli of the plant kingdom.’”
The breakthrough that really vaulted Arabidopsis closer to this lofty status came in 1989, when researchers first demonstrated that Agrobacterium’s T-DNA could systematically knock out genes. By inserting T-DNA randomly into the Arabidopsis genome, they could disrupt individual genes and create mutant plants lacking specific gene functions. This “reverse genetics approach” became the standard method for characterizing plant gene function and led directly to the creation of comprehensive mutant collections. The Salk Institute’s T-DNA collection and similar global resources now provide researchers with ready-made knockout mutants for nearly every Arabidopsis gene, making it possible to study gene function without generating new mutants from scratch.
By the early 90s, it appeared the transformation of Arabidopsis had occurred at two different levels — that of its DNA (now manipulable thanks to plant transformation) and that of its larger role within the research community. No longer an innocuous mustard varietal, Arabidopsis seemed a promising model organism. This was also the “Genome Era,” with the Human Genome Project well underway by 1990. Plant biology found itself swept up in the same current of excitement, enticed by the possibility of mapping a plant’s genetic blueprint.
Because Arabidopsis had one of the smallest and simplest plant genomes (~135 million bases across five chromosomes), researchers saw it as the ideal candidate for the first plant to be fully sequenced. While doubtlessly easier to sequence than a human genome, it still took roughly a decade to complete. The complete sequence was published in Nature in 2000 (four years after Baker’s yeast, the first eukaryotic genome, and one year before the human genome).
In the years that have elapsed, the value of Arabidopsis has only grown. For one, it has become much easier to work with. The year before the full genome sequence was published, researchers discovered the “floral dip method” in which flowering Arabidopsis plants are simply dipped into a solution containing Agrobacterium, allowing the bacteria to naturally transform developing seeds without any need for tissue culture. This breakthrough expanded the Arabidopsis research community dramatically. “Until other plants with fully sequenced genomes can be transformed with comparable ease, Arabidopsis is unlikely to be displaced as the plant of first choice for experimental molecular geneticists,” write Arabidopsis pioneers Chris Somerville and Maarten Koornneef in Nature.
A. thaliana’s primacy as a plant model, however, extends beyond molecular genetics; it’s been employed to decipher the genetic control of flowering, leaf and root formation, hormone signaling, circadian rhythms, and plant–pathogen interactions. And because many of its genes and pathways are conserved across plant species, insights gained from Arabidopsis routinely guide crop improvement and environmental research, making it a central reference species. It has even grown in space in an orbital laboratory, proving its resilience is not gravity-dependent.
In the end, Arabidopsis’s origin in Harz, a region renowned for its witchcraft, seems fitting. This thale cress is indeed a shapeshifter; once the passion project of a few dozen researchers, it has transformed itself into the laboratory’s most iconic plant.
Xander Balwit is editor-in-chief of Asimov Press and a Brassicaceae superfan.
Huge thanks to Evan Groover for providing feedback and clarifications on earlier versions of this draft. Header image by Ella Watkins-Dulaney, with credit to Marie-Lan Nguyen.
Thank you also to Dr. Marc Somssich, whose piece, Short History of Arabidopsis thaliana, clarified a lot of the plant’s early history and pioneers.
Cite: Balwit, X. “A Most Important Mustard.” Asimov Press (2025). https://doi.org/10.62211/83gr-98jh
Thal originally named it Pilosella siliquosa. However, it was renamed Arabis thaliana in honor of Thal by Carl Linnaeus in 1753.
He logged these plant observations in a posthumously published book with the delightfully prolix title of: The Hercynian Forest, or a catalogue of plants growing spontaneously in the mountains and most places of the Hercynian Forest which overlooks Saxony. It was published in 1588 by Thal’s brother-in-law, Joachim Camerarius the Younger, five years after Thal’s death.
Gerhard Röbbelen was an extremely prolific plant scientist who authored more than 400 papers over his research career. Beyond his pioneering work on Arabidopsis, he is internationally recognized for his work on improving the oil quality and yield in Oilseed rape, an important staple crop in Europe.