A Shift from Animal Testing
There has been a push toward animal-free alternatives in scientific research. But the success of such alternatives hinges upon whether and where they can outperform standard animal models.
Scientists have been using animals to study biology since the advent of Western science. Nearly 2,000 years ago, dissecting human corpses was taboo, but experimenting on living animals was so widely accepted that Galen’s public vivisections of squealing pigs in Ancient Rome drew crowds of distinguished guests and curious onlookers.
The animal testing industry, however, is much more recent.
Less than a century ago, American pharmaceutical companies could sell drugs without testing them at all. Drug makers weren't forced to do so until the passage of the Food, Drug, and Cosmetic Act in 1938. And the law only required them to demonstrate that their drugs were safe, not necessarily effective.
This laxity began to change in 1962 in the wake of the thalidomide disaster, in which a morning sickness medication was linked to severe birth defects in over 10,000 children worldwide. In response, U.S. lawmakers tightened drug regulation, and since then, drugmakers have been required to submit experimental data from at least two animal species before testing in humans.

Today, nearly all drugs and many of the cosmetics on the market were first tested on lab animals. Although the exact number of lab animals used today is contested, we know that roughly 852,000 animals covered by the Animal Welfare Act, including nonhuman primates, dogs, cats, and birds, were used last year. The number of rats and mice — the vast majority of lab animals — is estimated at over 100 million annually.
Animal testing faced strong backlash from its inception. The Greek philosopher and naturalist, Theophrastus, objected to animal dissections on the grounds that “causing pain to animals was an affront to the gods.”
Centuries later, Charles Darwin wrote his thoughts on vivisection to renowned morphologist Ray Lankester: “I quite agree that it is justifiable for real investigations on physiology; but not for mere damnable and detestable curiosity.”
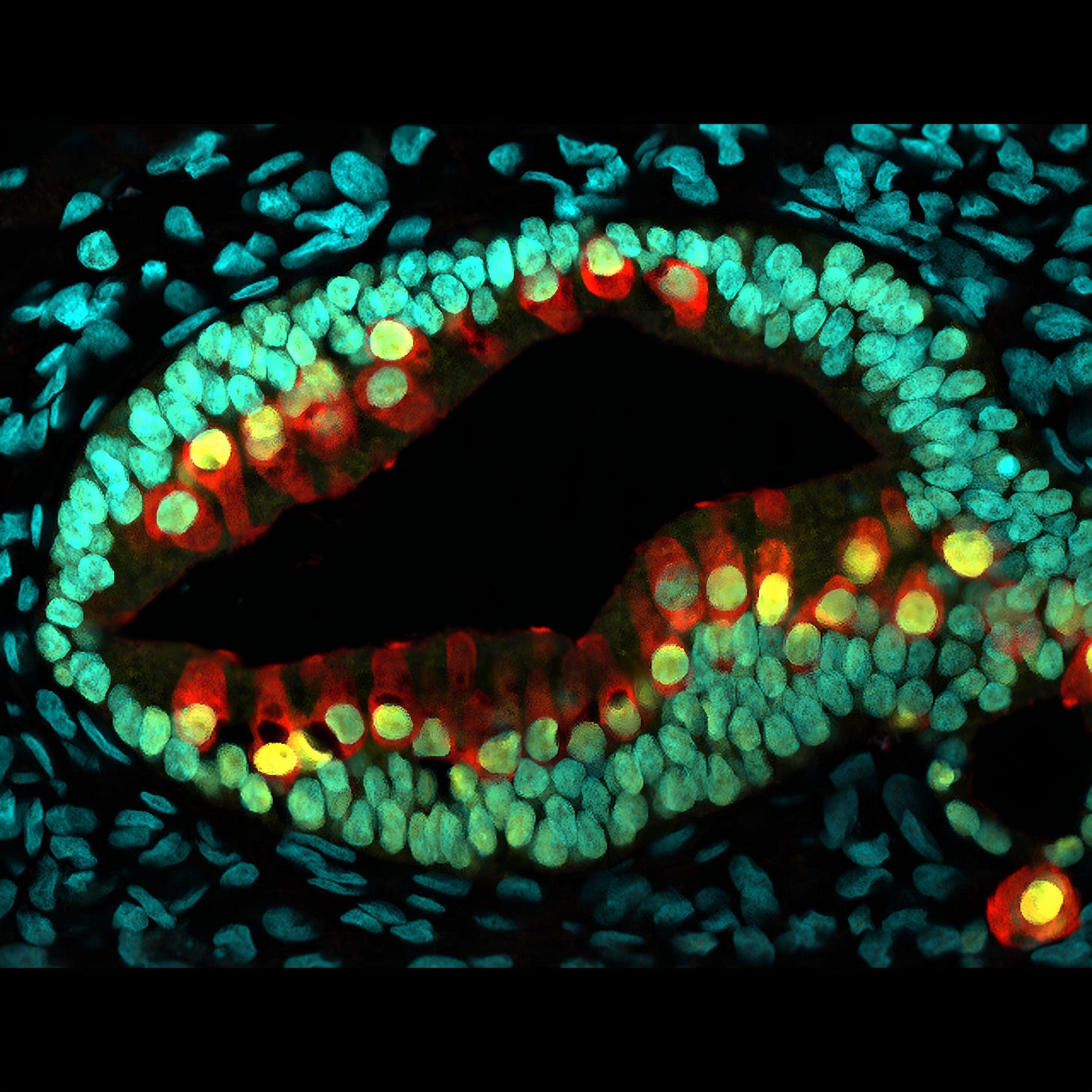
Like many in the scientific establishment, however, Darwin viewed animal testing as a necessary evil in the absence of viable alternatives. But this is beginning to change. Biotechnology innovations such as microfluidic chips, induced pluripotent stem cells, and 3D bioprinting are making it possible to grow human tissue for testing purposes, tailored to specific patient populations.
What’s more, shifting from animal testing is not only finally possible but may actually be required (if only in certain contexts). This year, both the U.S. Food and Drug Administration (FDA) and the National Institutes of Health (NIH) announced plans to reduce such testing. The FDA committed to making animal studies “the exception rather than the norm for pre-clinical safety/toxicity testing” over the next 3-5 years.
Meanwhile, the NIH is allegedly creating a new office to end animal-specific funding calls and expand funding and training for “New Approach Methodologies” (NAMs). In late July, the newly-launched Validation and Qualification Network, an initiative of the Foundation for the National Institutes of Health, partnered with major regulators and large pharmaceutical companies to get promising NAMs standardized and approved.

As encouraging as this shift may be, its success hinges upon whether and how well these animal-free alternatives actually work. Their utility is likely to play out differently across biomedicine. In some areas of biomedical research, such as safety screenings for shampoos or laundry detergents, petri dishes of human cells are already sufficient to determine whether a chemical is harmful or beneficial.
But in others, such as the search for treatments for neurological diseases, even the most advanced tools cannot accurately recapitulate the complexity of a living body. To truly transform the massive animal research industry, we’ll need to be honest about NAMs’ limitations — and our own.
The Limits of Animal Models
Even ancient physicians like Galen reasoned that the anatomical structures documented in human-like monkeys must be analogous to those of humans. In his lectures, De anatomicis administrationibus, Galen noted “the ape is likest man in viscera, muscles, arteries, veins, and nerves, as in the form of the bones.”
It wasn’t until DNA sequencing in the 20th century, however, that scientists could more rigorously establish just how similar we are to our animal relatives. Today, it is recognized that humans share roughly 90 percent of our genome with mice and even more with certain monkeys. This close genetic similarity seems to suggest that mice and monkeys would be good stand-ins for drug testing, but even small genetic differences can have huge, often unpredictable effects.
The truth is that animal studies rarely replicate in humans. One review article evaluating 221 animal experiments found that the results replicated in human studies just 50 percent of the time. A 2014 analysis of 2,366 drugs that had been tested in both humans and animals found that “results from tests on animals (specifically rat, mouse and rabbit models) are highly inconsistent predictors of toxic responses in humans, and are little better than what would result merely by chance — or tossing a coin — in providing a basis to decide whether a compound should proceed to testing in humans.”
The more data we amass about such biological idiosyncrasies, the tougher it is to justify directly translating findings from animals to humans. Researchers have dubbed this growing disconnect “the translation crisis” — a “valley of death” between promising animal studies and successful human treatments.
Even if species-specific quirks were few and far between (and they are not), lab animals generally make for homogeneous, distressed models of human health. Inbred mouse models such as C57BL/6 (the most widely-used of all mouse strains), for example, often spend their lives in small cages, stacked in colony room shelves, where they quarrel with cage-mates and develop heart disease. Rather than modeling the richness of human health and behavior, these mice are the equivalent of studying “20-year-old Californian white males who live by themselves and eat pizza all the time,” according to Lindsay Marshall, Director of Science at Humane World for Animals.
Perhaps unsurprisingly, then, most drugs — about 90 percent — tested on animals fail in human clinical trials. In the worst cases, patients die from organ failure; as happened during a phase II trial of fialuridine, a hepatitis B antiviral drug, in which 5 of 15 patients died from liver failure. (The molecule inserted itself into mitochondrial DNA, halting their replication and killing liver cells.)
In other trials, drugs that seemed to work in mice prove completely ineffective in humans (this happens time and time again with cancer treatments) or cause unexpected side effects, as when the antihistamine Seldane had to be pulled from the market in the late 1990s after some users experienced serious heart problems.
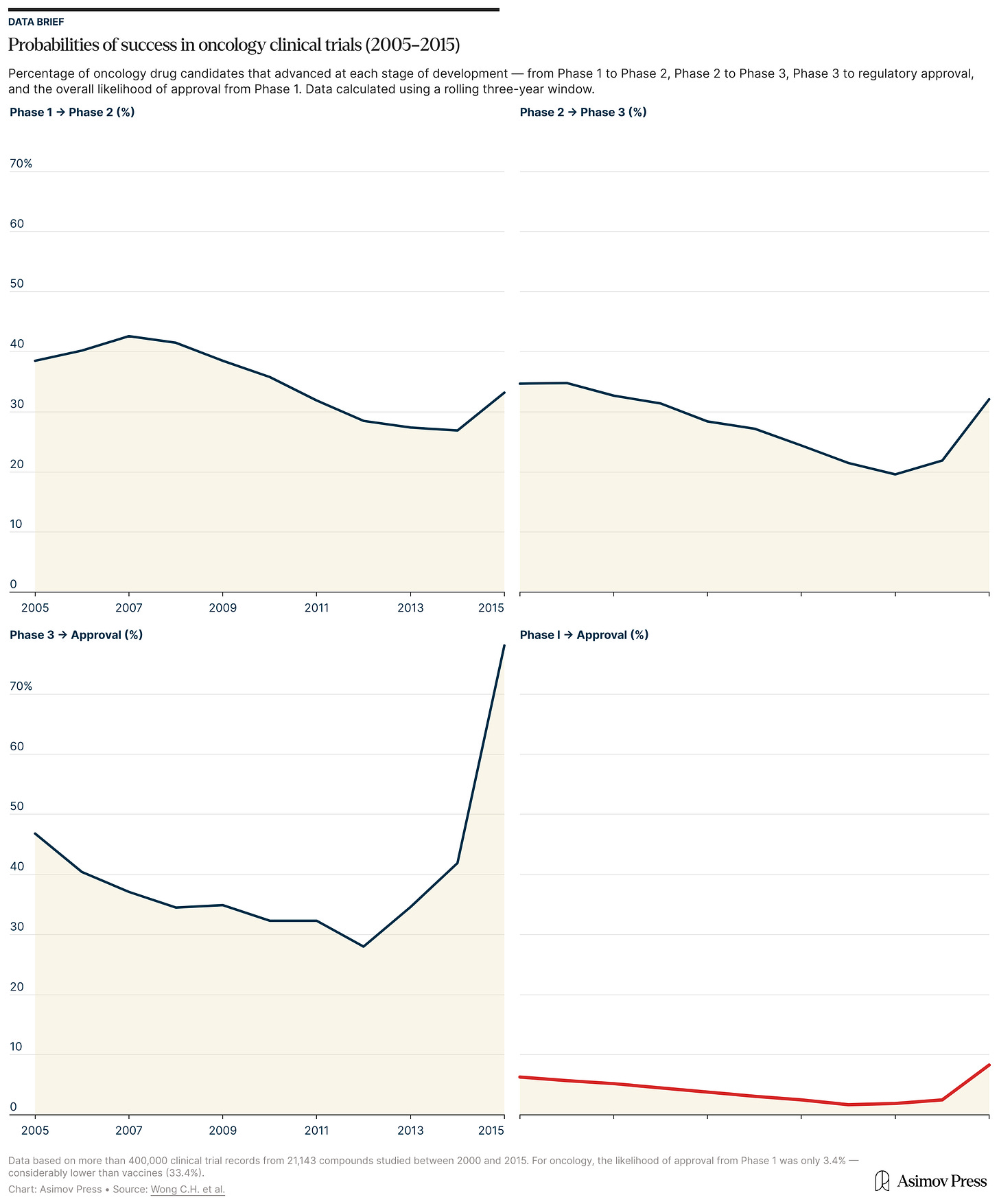
One 2024 review found that while roughly half of therapies advance from animal to human studies, only about five percent end up getting FDA approval. Success rates varied dramatically by disease type. About 20 percent of animal-tested cancer treatments made it to human clinical trials, but therapies for psychiatric disorders like anxiety and schizophrenia fared much worse (with none making it from RCTs to FDA approval).1
We can partially attribute such mixed results to accidents of history. After all, commonly used lab animals like mice and macaques were never fully validated before becoming the norm.2 Rather than being systematically tested to prove they accurately forecast human responses to medicine, these animals were chosen for more practical reasons: macaques resemble humans (especially in neurological studies and vaccine development), and mice are small, readily accessible, and easy to breed.
“We just got handed animal testing from historical use cases because that’s all they had in the fifties and sixties,” says Brandon White, co-founder of biotech startup, Axiom. But, “no one can really tell you its exact accuracy,” he added. “I think that’s a big problem.”
Despite these translational shortcomings, animal testing remains the norm due, in part, to inertia.
The high costs, long timelines, and high failure rates of preclinical trials have stalled biotechnology projects for years, leading to declines in investor interest and fewer late-stage clinical trials. But when the success of preclinical trials was first called into question, researchers rarely searched for answers outside monkeys or mice. Instead, they proposed a host of solutions within the existing paradigm of animal research: design more rigorous experiments, use better statistical tools, diversify lab mice, standardize housing conditions, and increase sample sizes. The logic was that if animal models couldn’t accurately predict human outcomes, there must be something wrong with the experimental design — not the model itself.
We can’t wholly fault them for drawing this conclusion. When preclinical animal studies fail to translate, serious design flaws are often to blame. For example, after AstraZeneca’s stroke medication NXY-059 flopped in human clinical trials despite promising preclinical results, a meta-analysis revealed that 60 percent of the animal studies weren’t properly randomized, and the tests used to evaluate the drug’s effects were inconsistent across studies.
Fixing methodological problems does usually improve studies. Properly randomizing and blinding preclinical animal studies (as is already expected in clinical trials) can prevent false positives and weed out ineffective drugs before they’re ever tested in humans. In the end, however, even a flawless study design can’t make monkey immune systems work more like ours, but better tool design might.
Establishing NAMs
In recent years, such tools have begun to proliferate, from miniature human organs grown outside the body to computer algorithms that improve predictions about a drug’s toxicity. These NAMS — “New Approach Methodologies” or “Non-Animal Methods,” depending on who you ask3 — range from simple chemical assays to complex 3D tissue models, each tailored to answer biological questions without relying on lab animals.
In cases where scientists know exactly how a biological process works, microplates loaded with molecules found in the body can be used to test whether a chemical is safe or not. Such in chemico techniques can show whether something will cause skin irritation, for example, and are already approved by the Organisation for Economic Co-operation and Development (OECD), which sets international standards for chemical safety testing.
In cases where researchers want to interrogate interactions between molecules within cells — like whether a chemical breaks down cell walls or damages DNA — they can grow a thin layer of cells on the bottom of a petri dish and run experiments in vitro. For many problems in chemical safety, like evaluating whether a new sunscreen causes inflammation or whether a pesticide triggers genetic mutations, this is sufficient. Many companies already trust in vitro tests, and major producers of shampoo, laundry detergent, and other household products have been relying on 2D cell cultures for decades.
In chemico and in vitro tests are generally cheap and easy, but a petri dish is a far cry from a mouse, and farther still from a human. A single sheet of cells cannot reproduce biological complexity when studying more complicated processes like tumor formation or liver toxicity.
For this, we must turn to 3D tissue cultures. Although scientists figured out how to culture cells in 3D over a century ago,4 it wasn’t until induced pluripotent stem cells were developed by Kazutoshi Takahashi and Shinya Yamanaka in 2006 that organoids — lab-grown blobs of self-organizing cells — became viable NAMs.
Organoids are made of stem cells, sometimes derived from a patient’s own tissue, artificially reprogrammed to become, say, neurons or cardiac tissue. Organoids can take on multiple roles, organizing themselves into sesame seed-sized mini-organs, scaled-down versions of their real-world counterparts. This allows them to model processes that flat sheets of cells simply can’t, like whether cancer drugs will shrink tumors, or how neurodevelopmental disorders emerge in growing brains.
But real organs aren’t just clusters of static cells — they’re complex tissues laced with blood vessels capable of drawing nutrients in and pumping waste out. Unlike organoids, next-generation “organ-on-a-chip” models, first introduced in 2010, pipe fluids through tiny channels lined with living human organ and blood vessel cells. Organ chips, each about the size of a thumb drive, mimic the natural flow of chemicals through tissue and can connect to other chips to simulate interactions between organ systems.
When researchers have amassed enough biological data about how these organ systems function, they can take the next step: modeling them computationally outside of the lab. Many drug companies already use mathematical models to predict how molecules will behave in the body. These models help them spot promising compounds buried in giant chemical databases.
Other computer programs simulate how a drug might be absorbed, distributed, and metabolized in a detailed model of the human body. For instance, if a researcher wanted to know how quickly the liver breaks down a new painkiller, they could input its molecular structure into a model that simulates liver enzymes, trained on data from previous real-world experiments.
Years before ChatGPT was released, researchers at Johns Hopkins Center for Alternatives to Animal Testing compiled millions of chemical structures from public databases to create a tool that outperforms traditional animal approaches for flagging toxic chemicals in consumer products. While they didn’t need the large language models powering today’s most advanced AI, LLMs can find hidden patterns in large datasets, which may accelerate drug discovery by months or even years.
Today, startups like Recursion are investing billions of dollars in AI. Others are spending hundreds of millions more commercializing NAMs, like Emulate’s organ chips or Vivodyne’s automated preclinical testing platform, which grows thousands of complex human tissues for testing at once. Realizing that NAMs can be faster, cheaper, and better at bringing new drugs to market than old-school animal testing, many companies have begun to explore them (even while continuing to primarily rely on animal experiments).
Two years ago, the pharmaceutical giant, Roche, launched the Institute of Human Biology, which focuses on engineering organoids and other human model systems. AstraZeneca and Johnson & Johnson have been using organ-chips and organoids for the past decade. Even Charles River Laboratories, a preeminent lab animal supplier, launched its own Alternative Methods Advancement Project in 2024. Through such programs, these companies are hoping to learn where NAMs actually work and where they may fall short.

Where Will NAMs Be Useful?
For animal rights advocates, the superiority of NAMs is obvious: If you believe that animals should not be used in science, even an imperfect alternative would be preferable. But for most researchers and the industry at large, “imperfect” isn’t nearly good enough, especially given that NAMs are meant as a corrective for the translation crisis. The key, then, is to establish just how good various NAMs are and for what.
Animals do have some traits that make them exceedingly useful as experimental subjects. As living creatures, they age (helpful in longitudinal studies), exhibit various behaviors allowing for the assessment of adverse reactions (such as vocalizations and nociception), and have complete physiological processes (such as immune systems). Without bodies, in vitro models can’t inform us about these embodied behavioral experiences.
For example, conditions like schizophrenia, autism spectrum disorder, and depression — all defined by subjective human experiences over time — can hardly be modeled in nonhuman animals, let alone isolated clumps of tissue. Even the scientists developing these tools have reservations. A 2024 review co-written by Dr. Fred “Rusty” Gage, a pioneer in using human stem cells to model brain disease, concluded that tissue models “will not replace nonhuman animal models or postmortem human brain tissue.”
But for questions of toxicity — what harm a substance might cause and how — several NAMs, even simple ones, are already at least as accurate as traditional animal studies. In the world of cosmetics testing, where the FDA never explicitly required animal testing, in vitro tests for skin and eye irritation have already largely replaced animals as the gold standard.
L'Oréal, for instance, reconstructs human skin to test whether its products cause allergic reactions and whether the active ingredients actually work. Unilever, the parent company behind brands like Dove and Axe, tests deodorant by spraying it in a mannequin’s armpits to see how much aerosol sticks, then tests whether those amounts irritate lab-grown human epithelial airway cells.
NAMs came more slowly to drug safety testing, where replacing animals wasn’t even an option until the FDA Modernization Act 2.0 took effect in 2023. However, in certain settings, effective animal-free alternatives existed for years before regulators formally approved them.

Take the pyrogen test, a procedure that detects whether a drug causes fever. Historically, this involved injecting the drug into rabbits and waiting for a rise in body temperature. Viable alternatives — monocyte activation tests — have existed since the mid-90s, where scientists directly expose human immune cells to fever-causing substances in a petri dish. After decades of advocacy, such tests are finally driving down the use of rabbits. And 25 years after this NAM was first validated, the rabbit pyrogen test was wiped from Europe’s official pharmaceutical guidelines.
As far as the underlying biology is concerned, testing for fever or skin irritation is relatively simple. Pyrogen tests probe a well-understood mechanism with a binary outcome: either immune cells release cytokines (the chemical messengers that trigger inflammation), or they don’t. But understanding how drugs are absorbed, metabolized, and spread throughout the body means grappling with the liver: a complex organ where multiple cell types interact to perform hundreds of different functions.
When you swallow a pill, drug molecules flow directly from your digestive tract to your liver before traveling anywhere else. Specialized enzymes metabolize the chemicals, but can only process so much before getting oversaturated. If more drug molecules enter the liver than it can handle, a backlog accumulates in liver cells and spills back into blood circulation — with potentially lethal consequences. When clinical trials fail or medications get pulled from pharmacy shelves, liver damage is often to blame, as was the case when the FDA withdrew the diabetes drug Rezulin back in 2000.
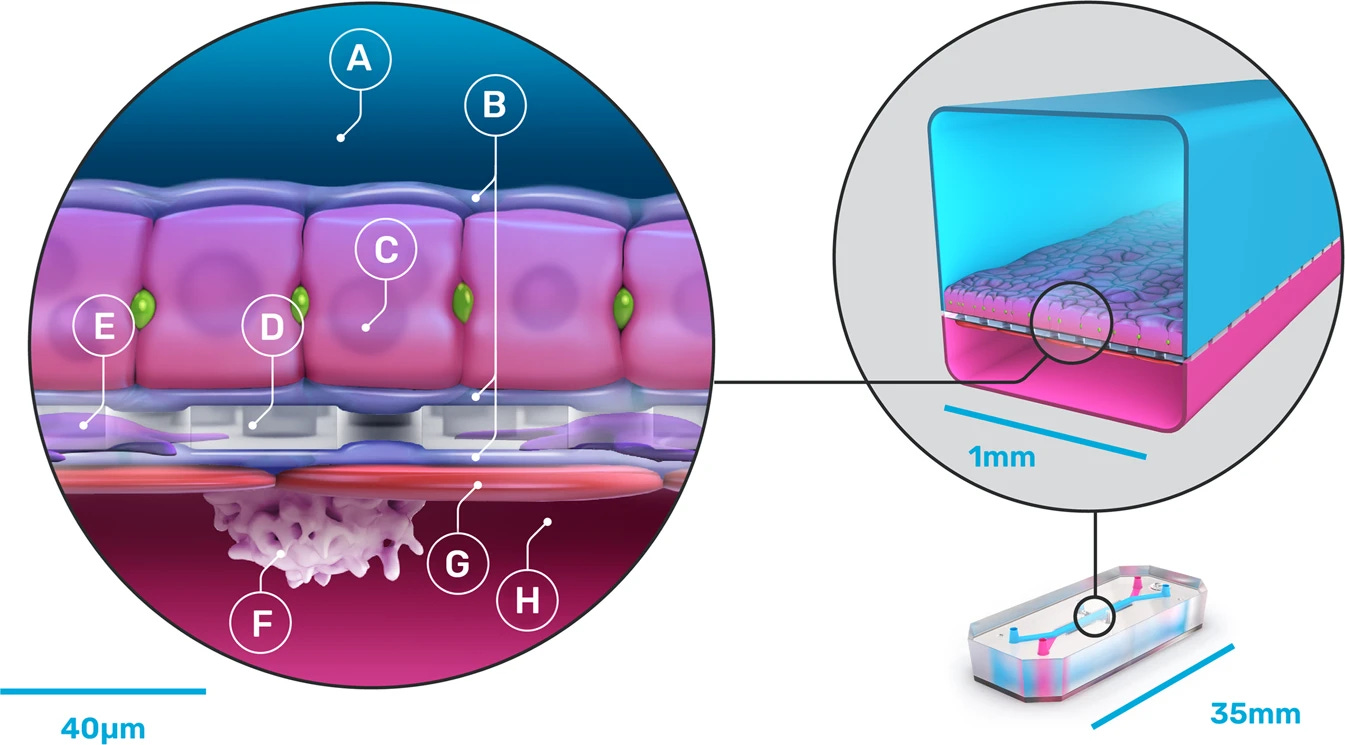
It’s no coincidence that some of the most promising animal alternatives targeted liver toxicity first. For example, Moderna uses “liver-chips” designed by Wyss Institute spinoff company, Emulate, to test their mRNA vaccines. (Specifically, to test the potentially toxic lipid nanoparticles, or LNPs, required to shuttle mRNA past the nuclear membrane.)
These specialized organ chips are embedded with crisscrossing porous channels: one filled with freshly-isolated human hepatocytes, which break down drugs and make proteins, and another with a blend of other liver cells involved in scar formation and inflammation. The use of these liver chips, instead of monkeys, allowed Moderna to screen potential LNPs in weeks, rather than an estimated 3.5 years, and save over $5 million.
Beyond toxicology, NAMs are already transforming the earliest stages of drug discovery. Traditionally, creating a new drug requires chemists to custom-build molecules that bind to specific disease-related proteins, which must then be tested and screened in living cells.
Instead, AI-driven startups like Axiom curate massive sets of training data that teach models to build and screen drug molecules. Their researchers imaged nearly 400 million liver cells after exposing them to over 115,000 different chemicals and combined these images with liver toxicity data scraped from tens of thousands of human clinical trials. CEO Brandon White hopes that their AI liver model will be able to replace preclinical animal tests to predict how dangerous new drugs might be for humans.
But this only works when there’s enough training data — and today’s AI models have a seemingly bottomless appetite. Manually labeling the images fed to Axiom’s liver model took over 7,000 hours, or nearly 3.5 years of full-time work. While those images were intentionally created as training data, the same can’t be said for the vast majority of biochemical data out in the world, much of which is messy, incomplete, or locked behind paywalls. When data is unusable or non-existent, it needs to be collected — often, still, from living animals.
Ultimately, well-defined questions with clear endpoints, like whether a cosmetic causes a rash or a drug triggers dangerous inflammation, can readily be answered without the use of animals. But the more complex and interconnected the biological question becomes, the more likely it is that NAMs fall short.
Selling Science on Animal Alternatives
Scientists will invest in NAMs only when they’re convinced that they work at least as well as the animal models they’re already comfortable with. Lorna Ewart, Emulate’s Chief Scientific Officer, said that, in many cases, companies still run animal studies alongside next-gen alternatives. “The technology,” she said, “is used ‘as well as’ rather than ‘instead of.’”
Emulate, for example, still validates their organ chips by comparing their results to monkey data collected in-house. When both sets of data align, it is a strong indicator that NAM data could meaningfully predict human health outcomes. However, such data cannot yet be exclusively relied upon. Even scientists who are eager to replace lab animals still view data from animal models as the “gold standard.”
With the launch of the Validation and Qualification Network (VQN) in July, promising tools may now have a clearer path to FDA approval. Now, a company making NAMs, such as brain organoids or AI liver toxicity models, can apply for VQN consideration, bringing their idea in front of a group of NAM developers, regulators, research organizations, and pharma companies.
If the network thinks their project is worth pursuing, they form a team that includes the NAM company, regulators, nonprofits, and, most importantly, the researchers who will actually use the NAM in practice. The team helps the company make sure that its NAM produces reliable, relevant data that researchers can use and regulators will trust.
However, the bar for trusting unfamiliar tools (especially those driven by AI) with critical decisions is extraordinarily high. Just look at autonomous vehicles: In the U.S., human drivers kill over 120 people every day, but a single fatal robotaxi accident got its manufacturer suspended. Similarly, regulators will need to decide how accurately AI must predict adverse events before it can be trusted above existing models.
Scientific validation isn’t enough, either. Even if all emerging NAMs are rigorously validated, perfectly predict human health outcomes, and link to clear-cut regulation, inertia will remain an impediment. “The evolution to a less animal-dependent future is as much a human psychology problem as it is a technical one,” said Brian Berridge, consultant and former Scientific Director of the National Institute of Environmental Health Science’s Division of Translational Toxicology.
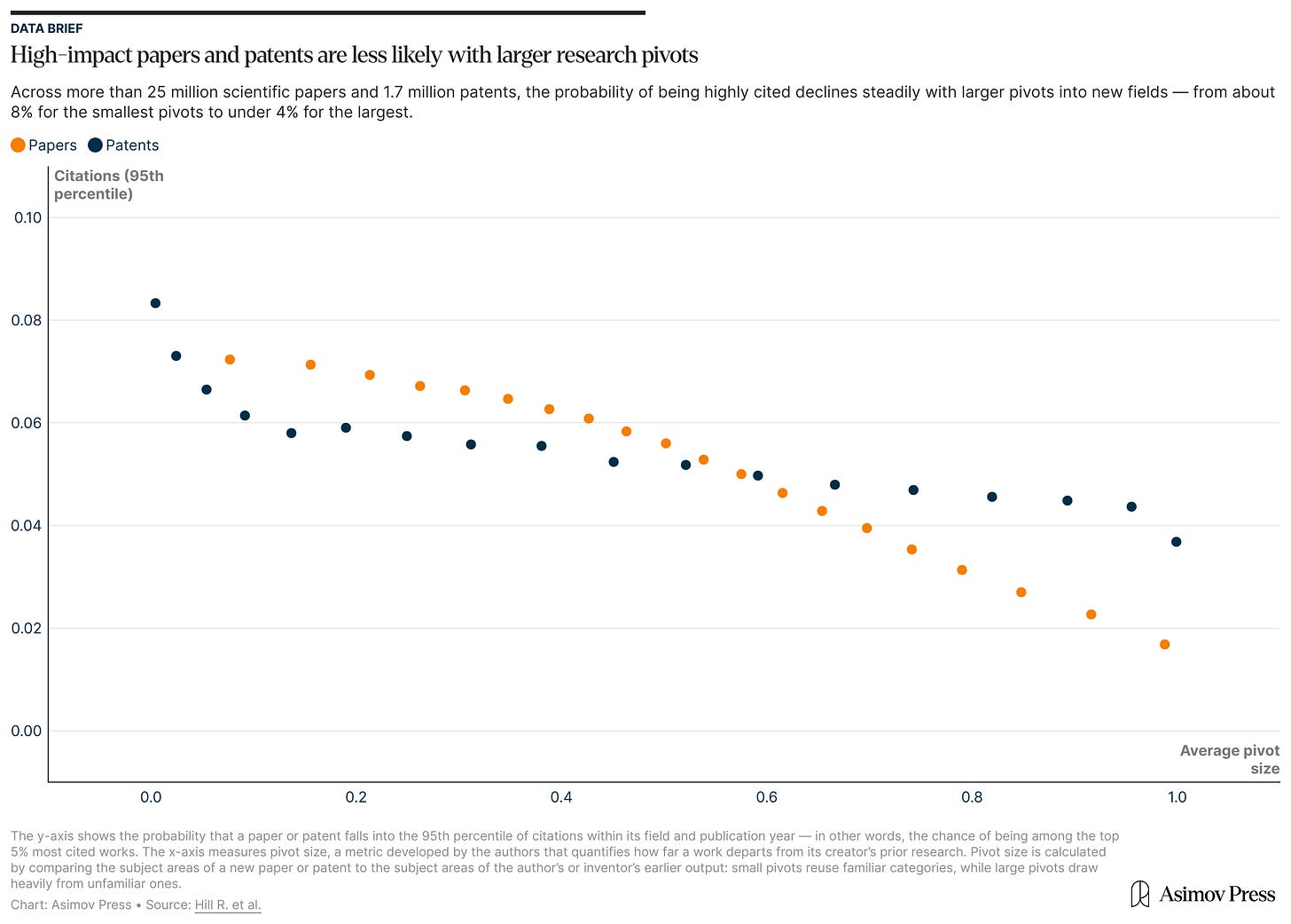
After all, scientists are often disincentivized from big pivots in their research program. Making important discoveries in science requires studying longer, working harder, and raising more grant money than it did in the past, and deviating from an already-established research track can jeopardize publication rates, tenure applications, and credibility. A paper published earlier this year reported that moving to a new research area lessens the impact of a scientist’s work, a phenomenon the authors said “applies nearly universally across science,” regardless of a scientist’s career stage, productivity, or funding situation.
Additionally, animal-based papers and grant applications are often reviewed by other animal researchers who aren’t comfortable evaluating studies using newer and less familiar models, including NAMs. Thus, excluding animal data risks ruining a lab’s already-slim chances of securing funding. There’s also some evidence that scientific journals are biased in favor of animal-based experiments, and that some researchers use animal methods as insurance against reviewers’ anticipated requests for it later.
Closely related to institutional inertia is the question of how the economics of NAMs might shake out. As expensive as animal research can be — a single rhesus macaque sells for tens of thousands of dollars (plus about that much per year to house) — NAMs aren’t necessarily cheaper, at least not initially.
Restructuring a lab to analyze 3D cell cultures can take years and cost hundreds of thousands of dollars upfront, excluding the price of retraining or hiring new staff. Meanwhile, a research facility with existing animal housing can keep a hundred mice alive for roughly $10,000 per year. As it stands, “there’s no incentive to spend more money, hoping that it gets you to a place where someday you can save money,” said Berridge.
Andrei Georgescu, CEO of biotech startup Vivodyne, argues that the real calculation isn’t about replacing cheap mice: It’s about avoiding expensive failures. Because clinical trial failures dwarf all other R&D costs, he suspects that pharmaceutical companies would happily pay “a million dollars per mouse” if each mouse could accurately predict which drugs would work in humans.
At Vivodyne, Georgescu’s team is building “maximalist” lab-grown tissue models at scale, using fleets of robots to generate hundreds of thousands of tissues complete with immune cells and blood vessels. By demonstrating that drugs have similar effects in their naturalistic tissue models and in humans, they’ve raised $40 million from venture capitalists eager to solve pharma’s animal testing problem. This private funding for a single startup dwarfs the NIH’s prize money for NAM development, which currently stands at just $1 million to be split across 20 teams.
Market forces, in fact, may wind up being the strongest driver of animal-free research. “In order to survive,” White said, private companies may “have to shift.”
This will be a challenge, however, given the Trump administration’s ongoing assault on American science. Government watchdog, White Coat Waste, gained traction under the first Trump administration by framing animal experimentation as a squandering of taxpayer dollars and continues to push hard against federally funded animal research. However, as federal agencies make bold commitments to reduce animal testing, Trump’s threats to punitively slash university budgets undermine the very institutions that produce biotech startups like Emulate and Vivodyne, and make it harder to train the scientists who would go on to staff such labs.
For animals to be removed from the lab as a “cost-saving measure,” researchers will need the infrastructure and support to develop alternatives. Scientists at public research institutions need more government backing — not less — to pivot away from the animal models they were trained on. Without money put towards retraining grants and NAM validation projects, years spent retooling their labs could cost some researchers their careers, killing momentum for animal-free research just as regulators are starting to take it seriously.
Breaking with the Past
Despite political headwinds, the FDA has come forward with a concrete plan to reduce animal testing, beginning with monoclonal antibodies: lab-made antibodies that grab onto particular proteins and block their functions.
Many of the best-selling drugs on the market today are monoclonal antibodies, including Humira and Keytruda, each with sales of about $21 billion in 2022 alone. Typically, developing a monoclonal antibody takes up to nine years, $750 million, and nearly 150 monkeys. But the gap between human and nonhuman immune systems is big enough that animal tests can’t accurately predict how the drugs act in humans. Considering their high costs and low risk of unexpected side effects, monoclonal antibody tests were an obvious starting point for the FDA. “They didn’t stick their neck out too far,” Berridge said.
However, if all goes well, these changes may soon extend to other drug categories, including other biologics and riskier small molecules.
That said, it is unlikely that the FDA’s proposed 3-5 year timeline for “making animal studies the exception rather than the norm” is feasible. More realistically, this will be the case for certain animals and only in certain contexts. Now that preclinical tests in two different animal species aren’t necessarily required, Ewart suggested that 3D tissue models, organ chips, and AI can start to replace animals that face strong bipartisan opposition, such as monkeys and dogs.
Berridge proposed continuing to use rodents as temporary “training wheels” alongside NAMs as the field begins to shift. Getting large animals out of labs sooner rather than later, with a longer-term goal of replacing small animals as well, “is a win that a lot of people can get behind,” Ewart said.
Still, by conceding that they can’t pull the plug on all animal testing overnight, the NIH has faced severe criticism from White Coat Waste, including online death threats targeting acting deputy director, Nicole Kleinstreuer.
Ambitious goals and uncompromising demands have their place, but there is also power in incremental progress, and in tackling specific, tractable problems with intention. Less than 50 years ago, testing cosmetics on animals was a universal norm. Today, advancements in animal-free toxicology and tireless animal rights campaigning have led to testing bans in 42 countries and counting (not including the United States).
Beyond cosmetics, many animal-free substitutes have become so commonplace that we barely remember their origins. Thanks to advancements in biotechnology, old practices like painstakingly purifying green fluorescent protein from bioluminescent jellyfish and grinding up pig pancreases for insulin have been largely replaced by synthetic techniques.

But these bioengineered alternatives wouldn’t exist without the animals that came before them, and science still grapples with questions that only living bodies can answer. By dismissing the value of animal research entirely, or denying that animal studies have contributed to biomedical breakthroughs, Berridge believes some activists hurt their own cause: “Anyone who recognizes the complexity of biology sees right through it.”
Phasing out animal models as better tools emerge is just the beginning. The trickier part will be confronting our own resistance to change. Old habits die hard in science, where sticking with familiar techniques often feels safer than experimenting with unproven alternatives.
Last year, Thomas Hartung, director of the Center for Alternatives to Animal Testing (CAAT), told me, "We can make progress, but sometimes progress takes an incredibly long time." Many toxicologists he knew were still using methods introduced when he — then 60 years old — was in kindergarten. Moving beyond these outdated approaches happens "one retirement at a time," Hartung explained.
Eventually, though, the next generation does move forward, pulling the rest of the field with them. Rapidly maturing NAM technologies, from AI models to organ chips, are beginning to align with regulatory momentum, venture capital investment, and enthusiasm from the pharmaceutical industry. While it is unclear exactly what this confluence will yield, it seems that meaningfully reducing animal testing — while still advancing human medicine — has never felt more possible.
Celia Ford is a journalist and ex-neuroscientist who writes about how technology shapes the creation of knowledge. Currently, she covers AI policy at Transformer and occasionally writes about alternatives to animal research at The Replacement. Previously, Celia completed reporting fellowships at Vox's Future Perfect, WIRED, and The Open Notebook. She is based in the California Bay Area and you can find her on X: @cogcelia.
Cite: Ford, Celia. “A Shift from Animal Testing.” Asimov Press (2025). DOI: https://doi.org/10.62211/37we-56kj
Lead image by Ella Watkins-Dulaney. Mouse background by Patrick Almhjell, PhD.
The complexity of the genetics underlying psychiatric conditions is partially responsible here. “In psychiatry…there have been virtually no examples of simple Mendelian inheritance and thus no single major genes have been discovered or exploited for developing a useful animal model of a common psychiatric disorder.”
Fully validating an animal model of disease would require comprehensively mapping how that species’s drug metabolism, drug receptor expression, immune responses, and disease pathways align with humans. This would involve cataloging gene expression, checking that disease models mirror human pathology, and running systematic cross-species studies to compare drug responses in animals and humans. But uncertainties introduced by species differences make it extremely challenging to confidently extrapolate results from animals to humans. One could even argue that if we had the technology to fully validate an animal model of disease, we probably would also have been able to develop NAMs to a point where the models are not necessary anyway.
The acronym “NAMs” originally referred to “New Approach Methodologies” — a broad category that included refined animal studies alongside animal-free alternatives. Today, advocacy groups use it more narrowly to mean “Non-Animal Methods.”
A 1907 paper from American biologist Ross Granville Harrison described an early method for culturing tissue in 3D: isolating bits of frog embryos and placing them in fresh frog lymph (lymphatic fluid that would act like culture media), which would clot around the tissue. The tissue could grow in this suspended position for over a week.


Really fascinatin to see CRL pivoting towards alternative methods while they're still the biggest lab animal supplier. Its like watching a company hedge their bets on both sides of the transition. Makes sense though, if NAMs are the future they want to be positioned for it rather than get left behind. The fact that they launced this in 2024 shows they're taking the regulatory shifts seriously.