Electronics Meet Microbes: Tiny 'Smart' Pill Senses Gut Inflammation
By merging bio-ware and hardware, Tim Lu's lab has developed a pill that can detect biomarkers of inflammatory bowel disease.
All disease begins in the gut.
Hippocrates, writing more than two millennia ago, may have been on to something. In recent years, a scientific renaissance has cemented a link between the gut microbiome and myriad diseases, including Alzheimer’s.
It’s no surprise, then, that many companies are creating therapeutics that promise to tweak the gut and, in turn, bolster health. Finch Therapeutics has already taken a C. difficile therapy into Phase 3 trials. Vedanta Biosciences is making drugs that are, themselves, built from microbial symbionts. And Synlogic has built engineered microbes that could help treat phenylketonuria and inflammatory diseases.
That last part — inflammatory diseases — is really important. Inflammatory bowel disease, or IBD, affects an estimated 3.1 million adults in the U.S. It’s an umbrella term that includes Crohn’s and ulcerative colitis. Sadly, adults with IBD have a higher risk of heart and lung disease, cancer, arthritis and ulcers, according to the Centers for Disease Control and Prevention.
Diagnosing IBD early, then, should be a top priority for researchers. Bioengineering is uniquely equipped to tackle the problem.
For a new study, researchers at MIT and Boston University made a pill that combines genetically-engineered microbes with electronic sensors. The result is a ‘living capsule’ that can detect molecules in the gastrointestinal tract, and then relay that information to a computer. The pill really works; it can detect a suite of inflammatory biomarkers in living animals, in about an hour.
A prior version of the pill, published in Science in 2019, could sense heme and inflammation markers in the gut. At the time, the researchers predicted they could reduce the volume “maybe by a third.” The new pill is more than six times smaller, with a total volume of 1.4 mL.
The paper, spearheaded by Rabia Tugce Yazicigil at Boston University and Tim Lu and Giovanni Traverso at MIT, was posted on bioRxiv last week.
UPSET STOMACH
The gut is a swirling sea of tension. It is filled with good and bad microbes, competing in an arms race of microscopic proportions.
Some of those microbes produce nitric oxide, which causes blood vessels in the gut's mucosa to shrink. This, in turn, lowers blood pressure. Hydrogen sulfide can oxidize n-butyrate, a good molecule that microbes produce, and impair colon function.
Both nitric oxide and hydrogen sulfide are short-lived molecules. They appear in the gut and disappear shortly after. Detecting them early, though, could help physicians diagnose and treat IBD.
There are really only two ways for a physician to peer inside the gut: Stick in a camera, or collect poop. The first is invasive and can damage gut tissue. The second is a bit like taking a picture of a party after the party has ended, and everyone has gone home. Bacteria in poop may not reflect bacteria living in the gut.
By developing a pill to track these compounds, the researchers have developed a tool that can non-invasively monitor these metabolites to better understand when and why they arise in patients, and what damage they really cause.
OINK, OINK
Design
The gut is a swirling sea of metabolites, many of which are structurally similar. It is often hard to detect one of these metabolites, like NO, without detecting another, like NOx.
The researchers made four biosensors. Each works in a similar way. A metabolite is first sensed by a protein (such as OxyR for hydroxen peroxide). The protein then switches on production of the bxb1 gene. This gene encodes a recombinase protein that inverts DNA sequences. In this case, when bxb1 is triggered, it switches a DNA sequence encoding green fluorescent protein, or GFP, and the cells glow green.

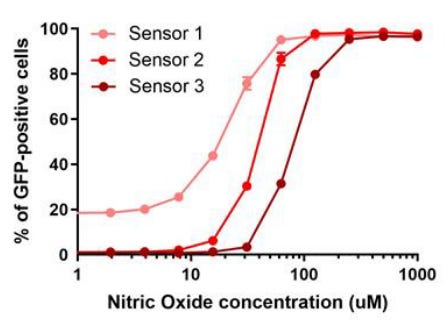
The NO biosensor can detect a concentration of just 30 μM diethylenetriamine/nitric oxide, and was not activated at all by other NOx compounds in the gut. The researchers also made three versions of the biosensor, each with a different detection threshold.
Validation
Mice were injected with dextran sodium sulfate, or DSS. This chemical induces inflammation in the GI tract. Mice treated with DSS are commonly used as a model for colitis, a disease in which the inner lining of the colon becomes inflamed.
The animals were fed each biosensor. Their poop was regularly collected and analyzed by flow cytometry. Animals given the NO biosensor had more GFP activation than control animals, meaning that the biosensor worked in living mice.

The biosensors took several days to work in mice. Tetrathionate biosensors had activated GFP about six days after the DSS treatment, tetrathionate after eight days and hydrogen peroxide after eight days. The biosensors were also successfully tested in pigs with intestinal inflammation.
Final Steps
Bacterial biosensors that detect disease biomarkers are nothing new. But they are unwieldy in many ways. If used in people, how would clinicians prevent the microbes from “leaking” into the world? And is it really feasible to collect and analyze poop every day?
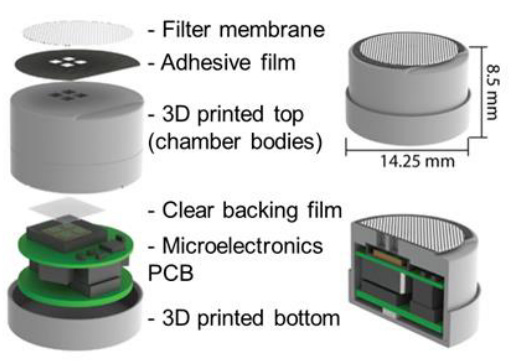
To make the biosensor more digestible, the researchers built a “bacterial-electronic pill,” just 1.4 mL in volume, with a special pocket for the microbes. The pill also has an electronic system that can detect photons and transmit a readout to a computer. The bacterial circuits were modified to switch on a luciferase operon, rather than GFP, to make them compatible with light-sensing electronics. The pills have an airtight seal; the microbes can’t escape.
A tiny sample of engineered bacteria (1 microliter), engineered to detect tetrathionate, were loaded into the pill’s chambers. The pill was fed to a pig with GI tract inflammation. The electronic system successfully detected the bacteria’s luciferase, recording “a net 175 fA photocurrent produced by the induced TT bacteria sensor.” The control animals did not have a measurable photocurrent.

This study is so appealing because it merges disciplines — synthetic biology and miniaturized electronics — to address a real-world need. The pills, too, are amazingly adaptable. Any microbe that can be engineered to detect a metabolite and produce light in response could theoretically be loaded into a pill and delivered to the gut for diagnostics. It all adds up to a new pill — six times smaller than its predecessor — that is easier to swallow. Ka-ching.
More soon,
— Niko



Do you think a similar approach can be developed for the detection of other diseases? Like in cancer detection can something similar replace biopsy?