Cable Bacteria are Living Batteries
How a discovery in a Danish lake changed our understanding of biological communities and energy.
Under a cloudless August sky, I sailed upon an InterCity train from København station to Aarhus, an 8th-century Viking settlement that is now the second largest city in Denmark. After disembarking, I trekked toward the local university to a door marked INSTITUT FOR BIOLOGI. Upon entering, I was greeted by Ian Marshall, a lanky Australian professor with graying hair, who ushered me into his first floor laboratory.
Dimly lit, the room was thick with the scent of wet earth. Large plastic buckets lined the walls, each filled with mud dredged from a nearby lake. Water-filled tanks sat on benches, bristling with a tangle of electrical probes and wires. Glass vials packed with dark sediment nestled in bubbling incubators. The room looked less like a biology lab and more like a cross between a hardware shop and a swamp; an engineered environment designed to coax electricity from mud.
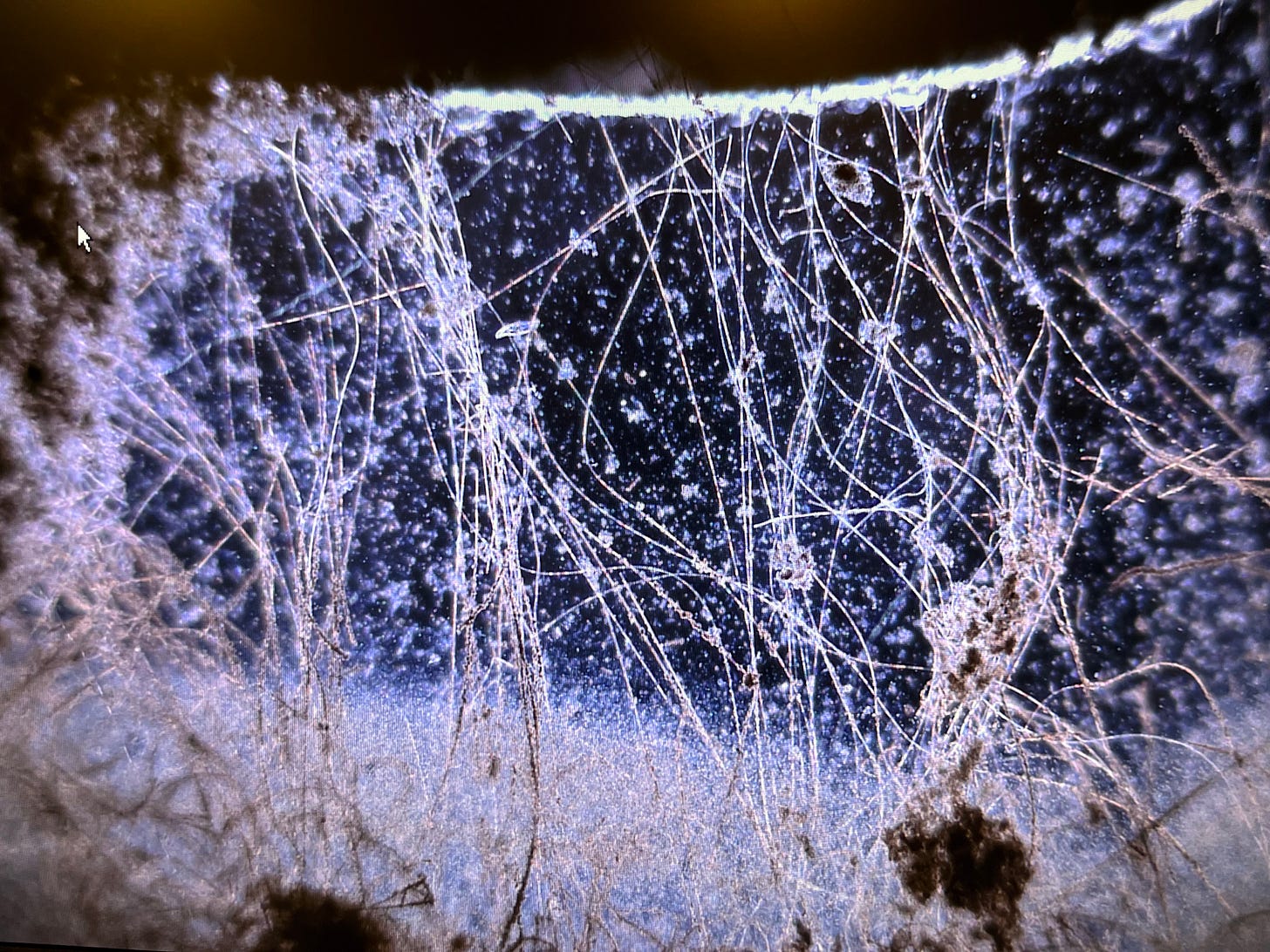
Marshall beckoned me to a microscope. Peering through the eyepiece, I saw fine, translucent filaments swaying gently against a dark backdrop. “What is this?” I asked. “It looks like hair.” Marshall chuckled.
“That’s them — the cable bacteria,” he said. “If you watch closely, you’ll see them twitching.”
I stared harder. The filaments shifted.
Although Aarhus University is not widely known outside Denmark (it wasn’t founded until the 1920s), it was here where scientists first identified the properties of the cable bacteria that I watched through Marshall’s microscope — a microbe that has revised much of what biologists know about bioenergetics.
A team of Aarhus scientists first discovered cable bacteria in a nearby lake in 2012. These microbes stack together, thousands of cells end to end, to form centimeters-long chains. One tip of this microbial chain oxidizes sulfide in the mud, stripping it of electrons. Once freed, those electrons then race up the chain toward the water’s surface, where a cell at the chain’s tip passes them to an acceptor molecule, such as oxygen or nitrate. Cable bacteria function, effectively, as living batteries.
The cells separate their redox chemistry (the cornerstone of energy generation across all lifeforms) across thousands of cells for a simple reason: In lakes and ponds, oxygen only penetrates the upper few millimeters of mud. Below that, sediment quickly turns anoxic, or oxygen starved. And because the microbes living deeper down cannot access oxygen, they must find another way to get rid of their electrons.
By creating this bridge, then, cable bacteria have evolved a clever way to separate the two halves of a redox reaction across a large physical space. They gain access to electron donors deep in the mud (like sulfide) and electron acceptors in the waters above (like oxygen). Not only are these microbes a marvel of cellular architecture, but they also stand in contrast to the long-held idea that cells only power themselves using local chemistry.
Indeed, nearly a century ago, the Dutch microbiologist Albert Jan Kluyver wrote: “From the point of view of energy metabolism, there is only one biology.” He meant that all life, from microbes to humans, relies on electron transfer from donor to acceptor. Cable bacteria obey this rule, but they stretch it in ways we never thought possible. They are bacteria, but they coordinate like tissues and behave like circuits.

The discovery of cable bacteria also came about partly by accident.
In the late 2000s, a Danish scientist named Lars Peter Nielsen collected some mud from a nearby lake (an eight-minute drive away from the university), placed it into glass beakers, and used tiny sensors to study the mud’s chemistry. According to a news story in Science:
At the start of the experiment, the muck was saturated with hydrogen sulfide — the source of the sediment's stink and color. But 30 days later, one band of mud had become paler, suggesting some hydrogen sulfide had gone missing. Eventually, the microsensors indicated that all of the compound had disappeared. Given what scientists knew about the biogeochemistry of mud, recalls Nielsen, who works at Aarhus University, "This didn't make sense at all.”
More specifically, this didn’t “make sense” because hydrogen sulfide is usually found deep in the sediment, where there’s no oxygen. In those conditions, most bacteria can’t metabolize it. The reaction that consumes hydrogen sulfide typically requires oxygen or another electron acceptor, and none should be available that far down. So when the sensors showed that the hydrogen sulfide had vanished, it seemed chemically impossible.
Oddly enough, when Nielsen stuck a thin wire into the sediment, it broke the pattern; the hydrogen sulfide stayed in place. He began suspecting that a special type of bacterium might be stripping electrons from the hydrogen sulfide and transporting them over vast distances. But when Nielsen mentioned this idea to colleagues, they were skeptical:
Filip Meysman, a chemical engineer at the University of Antwerp, recalls thinking, "This is complete nonsense." Yes, researchers knew bacteria could conduct electricity, but not over the distances Nielsen was suggesting. It was "as if our own metabolic processes would have an effect 18 kilometers away," says microbiologist Andreas Teske…
Despite the skepticism, Nielsen published his results in Nature in 2010. Two years later, he conclusively showed that his hunch about microbes, or “cable” bacteria, was correct.
Since then, researchers have learned a great deal more about how cable bacteria move electrons across distances of up to 7 centimeters (a length 30,000-times further than a single bacterium). Each bacterium in the cable has ridges, made from proteins, embedded in the space between its inner and outer cell membranes. These ridges act like buried wires that carry the electrons. At the place where two cells in the chain meet, these protein wires move from one cell’s periplasm to the next, thus forming a continuous, conductive path up the entire filament.
And while the discovery of cable bacteria may in itself be a marvel of basic research, the bacteria’s ability to shuttle electrons also has practical uses. For a 2020 study, researchers added cable bacteria to soil pots planted with rice and then tracked methane emissions, soil chemistry, and plant growth over multiple weeks. Because these microbes strip electrons from sulfide in the mud, they generate lots of sulfate, raising its concentration to five times that of controls. This spike in sulfate suppressed methane-producing microbes, known as methanogens, cutting methane emissions by 93 percent.
Given that rice agriculture alone accounts for about 11 percent of human-driven methane emissions, adding cable bacteria to rice paddies could have an enormous positive impact on the environment. Field trials are now being carried out in California to figure out whether these methane reductions will hold up under more realistic farming scenarios. Researchers are also trying to learn whether wheat, barley, and other major cereals would see a similar reduction in methane emissions after the addition of cable bacteria.
Unfortunately, using cable bacteria in the real world is hamstrung by the fact that these cells cannot yet be grown in pure culture or be genetically engineered. Cable bacteria only grow in mud and water with chemical gradients. The cells also divide slowly — about once every 20 hours — and nobody has figured out a way to get DNA into them, in part because of their thick, cellular membranes.
Back in the lab, I pulled my eyes away from the microscope. Marshall ushered me up twisting corridors to the building’s top-floor cafeteria. Over potatoes and Frikadeller (Danish meatballs), I asked Marshall what puzzled him most about these organisms. He paused for a long time, then said: “Everything.” Despite gains in our understanding of them, cable bacteria still defy easy explanation.
I looked up from my plate and gazed through a window. A lake shimmered in the distance.
Niko McCarty is a founding editor at Asimov Press.
Cite: McCarty, N. “Cable Bacteria are Living Batteries.” Asimov Press (2025). https://doi.org/10.62211/52jg-21uo